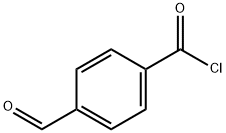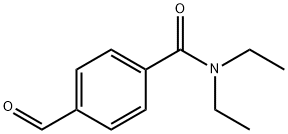
N,N-diethyl-4-forMylbenzaMide synthesis
- Product Name:N,N-diethyl-4-forMylbenzaMide
- CAS Number:58287-77-7
- Molecular formula:C12H15NO2
- Molecular Weight:205.25
Yield:58287-77-7 90%
Reaction Conditions:
Stage #1: 4-Carboxybenzaldehydewith thionyl chloride in toluene at 60;
Stage #2: diethylamine in dichloromethane at 0; for 2 h;Heating / reflux;
Steps:
1 INTERMEDIATE 1: N, N-DIETHYL-4-FORMYLBENZAMIDE
To a suspension of 4-carboxybenzaldehyde (30 g, 0.2 mole) in 100 ml of toluene was added SOC12 (97 ml, 1.3 moles) at 60 °C. The reaction was heated until gas evolution ceased followed by evaporation to dryness with toluene (3 x 50 ML) This yielded a residue, which was dissolved in CH2C12 (200 ML). To this solution, cooled in an ice bath while stirring, was added diethylamine (50 mL). Stirring was continued for one hour and then the mixture heated at reflux for a further hour. After cooling, the mixture was washed successively with H2O, 2 N HC1, HA0 then 2 N NAOH and finally with H20. The solution was dried over MGS04, filtered and concentrated to dryness yielding 41 g of oil. Distillation at 140-150 °C/1. 5 torr gave 36.9 g, 90 % of INTERMEDIATE 1.
References:
WO2004/41800,2004,A1 Location in patent:Page 27

619-66-9
412 suppliers
$5.00/5g

58287-77-7
10 suppliers
inquiry
3282-30-2
356 suppliers
$19.85/100ml

619-66-9
412 suppliers
$5.00/5g

109-89-7
449 suppliers
$10.00/5g
121-44-8
835 suppliers
$9.00/5g

58287-77-7
10 suppliers
inquiry

619-66-9
412 suppliers
$5.00/5g

109-89-7
449 suppliers
$10.00/5g

543-27-1
246 suppliers
$10.00/5g

58287-77-7
10 suppliers
inquiry