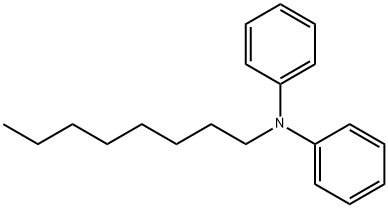
N-octyl-N-phenylaniline synthesis
- Product Name:N-octyl-N-phenylaniline
- CAS Number:86-25-9
- Molecular formula:C20H27N
- Molecular Weight:281.44
Yield:86-25-9 87%
Reaction Conditions:
with N-benzyl-N,N,N-triethylammonium chloride;sodium hydroxide in water; for 24 h;Reflux;
Steps:
2.1 2.2.1. N-octyldiphenylamine (1)
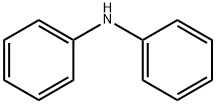
A mixture of diphenylamine (10 g, 0.056 mol), octyl bromide(12 g, 0.062 mol), benzyltriethylammonium chloride (BTEAC) (1.4 g,6.0 mmol), NaOH (12 g, 0.3 mol) and H2O (10 mL) was vigorouslystirred for 24 h at reflux temperature (Scheme 1). After cooling, the organic layer was separated. The crude product was purified bycolumn chromatography on alumina (elution with petroleumether). Yield 14.4 g (87%), colorless oil. 1H NMR (CDCl3, 400 MHz) δ:0.88 (3H, t, J 6.9 Hz, Me), 1.28 (10H, m, (CH2)5), 1.66 (2H, m, CH2),3.68 (CH, t, J 7.8 Hz, NCH2), 6.94 (2H, m, HAr), 6.99 (4H, m, HAr), 7.26(4H, m, HAr) ppm (1H NMR Ref. [38]).
References:
Baranov, Denis S.;Uvarov, Mikhail N.;Kazantsev, Maxim S.;Mostovich, Evgeny A.;Glebov, Evgeni M.;Gatilov, Yurii V.;Kulik, Leonid V. [Dyes and Pigments,2017,vol. 136,p. 707 - 714]

111-86-4
371 suppliers
$5.00/25g
108-90-7
643 suppliers
$10.00/25G
3007-71-4
12 suppliers
inquiry

86-25-9
6 suppliers
inquiry

111-86-4
371 suppliers
$5.00/25g
100-68-5
336 suppliers
$9.00/1g

3007-71-4
12 suppliers
inquiry

86-25-9
6 suppliers
inquiry

111-83-1
493 suppliers
$12.00/25g
5856-89-3
0 suppliers
inquiry

111-66-0
145 suppliers
$10.00/10ml

86-25-9
6 suppliers
inquiry