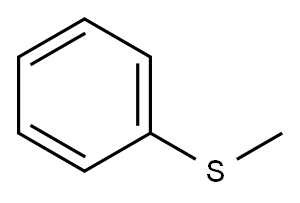
Thioanisole synthesis
- Product Name:Thioanisole
- CAS Number:100-68-5
- Molecular formula:C7H8S
- Molecular Weight:124.2
Yield:100-68-5 99%
Reaction Conditions:
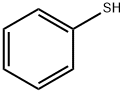
Stage #1: thiophenolwith sodium hydroxide in ethanol; for 0.0833333 h;
Stage #2: methyl iodide in ethanol at 20; for 5 h;
Steps:
3.1. General Procedure in Alkylation of Aryl Thiol
General procedure: To a solution of NaOH (11 mmol) in EtOH (1 mL), thiophenol 6 (9.1 mmol) was added. After 5 min of stirring, alkylhalide (11 mmol) was added drop-wise and the mixture was left stirring for 5 h at room temperature. The reaction was quenched with H2O (10 mL) and the aqueous solution was extracted with ethyl acetate (3 × 10 mL). The combined organic fractions were dried over MgSO4 and concentrated in vacuo under reduced pressure. The residue product was purified using column chromatography on silica gel using 100% hexane as eluent to afford the title compounds: Methyl(phenyl)sulfane (7a): Colorless oil; 99%; 1HNMR (CDCl3, 400 MHz): δ = 7.32-7.26 (3H, m, Ar), 7.15(2H, t, J = 12.0 Hz, Ar), 2.49 (3H, s, -SCH3); 13C (CDCl3,100 MHz): δ = 138.3, 128.7, 126.5, 124.9 (C, -Ar), 15.7 (C, -SCH3). The spectroscopic data were in agreement with theliterature report [25].
References:
Mabasa, Tommy Fredrick;Awe, Babatunde;Laming, Dustin;Kinfe, Henok Hadgu [Medicinal Chemistry,2019,vol. 15,# 6,p. 683 - 690]
1193-82-4
132 suppliers
$13.00/1g

100-68-5
354 suppliers
$9.00/1g
104-95-0
219 suppliers
$8.00/5g

100-68-5
354 suppliers
$9.00/1g