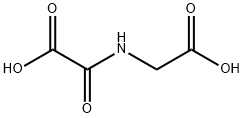
N-OXALYL GLYCINE synthesis
- Product Name:N-OXALYL GLYCINE
- CAS Number:5262-39-5
- Molecular formula:C4H5NO5
- Molecular Weight:147.09
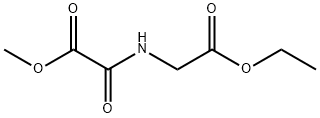
1365536-49-7
0 suppliers
inquiry

5262-39-5
105 suppliers
$25.00/5mg
Yield: 99%
Reaction Conditions:
with potassium hydroxide in methanol at 60;Inert atmosphere;
Steps:
10.13 4.10.13. N-Oxalylglycine (2)
N-(Methoxyoxalyl)glycine ethyl ester (100 mg, 0.53 mmoles) and KOH (88 mg, 1.6 mmoles) were added to a vial. MeOH (5.0 mL) was added to the vial, and the resulting reaction mixture was heated to 60 C until the starting material was consumed completely,as judged by TLC. The reaction mixture was cooled to roomtemperature and concentrated under reduced pressure. The crudeproduct was dissolved in water (2 mL), and the pH of the resultingsolution was adjusted to 3-4 with 1 M HCl. The aqueous solutionwas concentrated under reduced pressure, and the residue was dissolvedin boiling EtOAc. The solution was cooled, filtered, and concentratedunder reduced pressure to a viscous oil. The oil wasdissolved in a minimal amount of DCM, diluted with hexanes,and concentrated under reduced pressure. This procedure wasrepeated 3 times, after which the product was dried under highvacuum to afford 2 (77 mg, 99%) as a white solid with mp 104.5-110.5 C. 1H NMR (500 MHz, DMSO-d6, d): 14.03 (bs, 1H), 12.76(bs, 1H), 9.04 (t, J = 6.0 Hz, 1H), 3.79 (d, J = 6.5 Hz, 2H); 13C NMR(125 MHz, DMSO-d6, d): 170.8, 162.1, 158.9, 41.4; HRMS (ESI)m/z 165.0506 [calc’d for C4H9N2O2 (M+NH4)+ 165.0506].
References:
Vasta, James D.;Raines, Ronald T. [Bioorganic and Medicinal Chemistry,2015,vol. 23,# 13,art. no. 12297,p. 3081 - 3090]
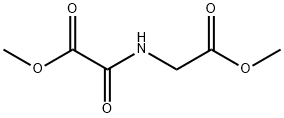
89464-63-1
161 suppliers
$14.00/10mg

5262-39-5
105 suppliers
$25.00/5mg
![Ethanamine, N-[(3,5-dichlorophenyl)methylene]-2,2-diethoxy-](/CAS/20210305/GIF/1000210-73-0.gif)
1000210-73-0
0 suppliers
inquiry

5262-39-5
105 suppliers
$25.00/5mg
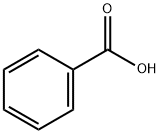
65-85-0
1422 suppliers
$10.00/25 g

556-50-3
530 suppliers
$15.00/25g

5262-39-5
105 suppliers
$25.00/5mg

556-50-3
530 suppliers
$15.00/25g

202650-88-2
16 suppliers
inquiry

5262-39-5
105 suppliers
$25.00/5mg