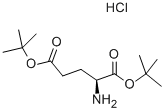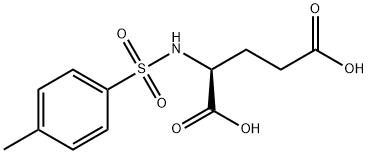
N-(p-Tolylsulphonyl)-L-glutamic acid synthesis
- Product Name:N-(p-Tolylsulphonyl)-L-glutamic acid
- CAS Number:4816-80-2
- Molecular formula:C12H15NO6S
- Molecular Weight:301.32
Yield: 92%
Reaction Conditions:
Stage #1:L-glutamic acid;p-toluenesulfonyl chloride with sodium hydroxide in water at 70; for 1.5 h;
Stage #2: with hydrogenchloride in water; pH=3 at 0;
Steps:
3
14.7 g (0.1 mol) L-glutamic acid was dissolved in 100 mL 2N NaOH, heated to about 70° C. in a water bath, then 22.8 g (0.12 mol) p-tosylchloride was added in portions under stirring over about half an hour. NaOH solution was added dropwisely continuously to keep pH at and the reaction was performed at 70° C. under stirring for 1 hour. After being cooled to room temperature, the reaction is cooled in an ice-salt bath to below 0° C., a concentrated hydrochloric acid was added dropwisely until pH was about 3, and extracted with 300 mL ethyl acetate in three portions. All extracts were combined, heated under reflux, decolored with active carbon, filtrated, dried over anhydrous calcium chloride as desiccant, filtrated again, and the desiccant was washed with anhydrous ethyl acetate. The obtained solution was concentrated to 80 mL, and cooled in an ice-salt bath to precipitate crystalline L-(+)-N-p-tosylglutamic acid of 27.7 g in a yield of 92%. [α]D20=+22.6 (room temperature, ethyl acetate, c=1.10), melting point: 130-132° C.
References:
INSTITUTE OF PHARMACOLOGY AND TOXICOLOGY ACADEMY OF MILITARY MEDICAL SCIENCES P.L.A.CHINA US2011/306633, 2011, A1 Location in patent:Page/Page column 4

56-86-0
791 suppliers
$5.00/25g

4816-80-2
135 suppliers
$23.60/25g
![Ethanamine, N-[(3,5-dichlorophenyl)methylene]-2,2-diethoxy-](/CAS/20210305/GIF/1000210-73-0.gif)
1000210-73-0
0 suppliers
inquiry

4816-80-2
135 suppliers
$23.60/25g