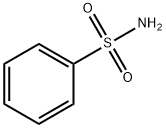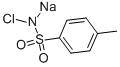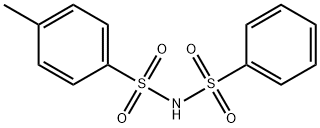
N-(phenylsulphonyl)-p-toluenesulphonamide synthesis
- Product Name:N-(phenylsulphonyl)-p-toluenesulphonamide
- CAS Number:14706-41-3
- Molecular formula:C13H13NO4S2
- Molecular Weight:311.3766
Yield:14706-41-3 52%
Reaction Conditions:
with 4‐dimethylaminopyridine;triethylamine in dichloromethane at 50; for 10 h;
Steps:
1.1. Prepare diarylsulfonimides 2
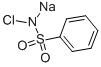
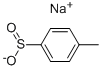
Typically, benzenesulfonamide (1.93 g, 12.3 mmol), p-toluenesulfonyl chloride (2.13 g, 11.2 mmol), DMAP (0.27g, 0.2 equiv) and DCM (30 mL) were added into a 100 mL double-necked flask equipped with a magnetic stirrer. Next, triethylamine (1.95 mL, 14 mmol) was added to the reaction solution. The mixture was heated to reflux at 50 oC for 10 h and cooled to room temperature. The reaction solution was quenched with 1 mol/L HCl (30 mL), and extracted with DCM (30 mL × 3), and then concentrated. The obtained crude product and 40 mL saturated NaHCO3 solution were added into a 100 mL flask. The mixture was heated at 100 oC for 1.5 h, and then cooled to room temperature. The pH value of the reaction solution was adjusted to 7 with 1 mol/L HCl solution. After filtration, the filtrate was retained and continued to be acidified to pH=1. After filtration, the filter cake was dried to obtain 1.82 g of product 2b in a yield of 52%, which was a white solid
References:
Liu, Zhiyuan;Wu, Wenliang;Yang, Jiangyu;Li, Meichao;Hu, Xinquan;Hu, Baoxiang;Jin, Liqun;Sun, Nan;Shen, Zhenlu [Tetrahedron,2022,vol. 119,art. no. 132853]