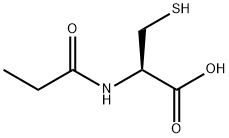
N-Propionyl-L-cysteine synthesis
- Product Name:N-Propionyl-L-cysteine
- CAS Number:2885-79-2
- Molecular formula:C6H11NO3S
- Molecular Weight:177.22
Yield:2885-79-2 1 g
Reaction Conditions:
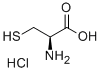
Stage #1: L-Cysteinewith sodium acetate in tetrahydrofuran;water at 20; for 0.333333 h;
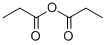
Stage #2: propionic acid anhydride in tetrahydrofuran;water at 5;Reflux;
Steps:
3 Example 3 - synthesis of (R)-4-(2-carboxy-2-propionamidoethylthio)-4- oxobutanoic acid (NV-041 , 01-041)
To a mixture of L-cysteine (2.00 g, 16.5 mmol) in THF/H20 (8 ml_/2 ml_) was added NaOAc (2.70 g, 33.0 mmol). The mixture was stirred at room temperature for 20 min. The reaction was cooled to 5 °C before propionic anhydride (2.30 g, 17.6 mmol) was added dropwise. The reaction mixture was stirred at room temperature overnight and then heated to reflux for 4 hours. The reaction mixture was cooled and acidified to pH 5 by adding 4N HCI. The resulting solution was evaporated under reduced pressure to remove THF. The residue was purified by prep-HPLC (eluting with H20 (0.05% TFA) and CH3CN) to give 1 .00 g of (R)-3-mercapto-2-propionamidopropanoic acid as colourless oil.
References:
WO2015/155231,2015,A1 Location in patent:Page/Page column 63; 64