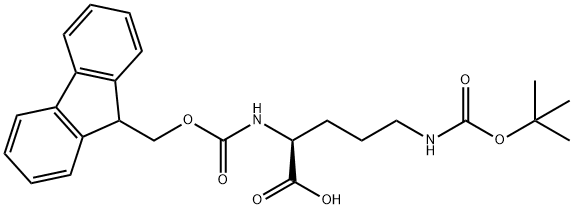
Fmoc-Orn(Boc)-OH synthesis
- Product Name:Fmoc-Orn(Boc)-OH
- CAS Number:109425-55-0
- Molecular formula:C25H30N2O6
- Molecular Weight:454.52
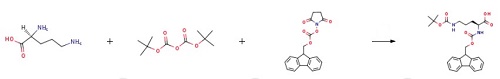
70-26-8
218 suppliers
inquiry

24424-99-5
824 suppliers
$13.50/25G
82911-69-1
605 suppliers
$7.00/25g

109425-55-0
311 suppliers
$8.00/1g
Yield:-
Reaction Conditions:
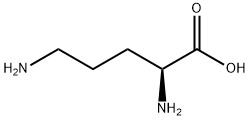
Stage #1: L-ornithine;di-tert-butyl dicarbonatewith 8-quinolinol;sodium hydrogencarbonate;sodium carbonate in water;acetonitrile at 20; for 4 h;
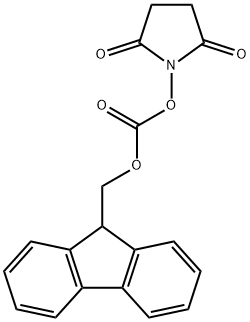
Stage #2: N-(9H-fluoren-2-ylmethoxycarbonyloxy)succinimide in water;acetonitrile at 20; for 2 h;Temperature;
Steps:
1.1 Example 1
2 mmol L-Orn was weighted and dissolved in 15 ml of acetonitrile; 16 mmol Boc2O was dissolved in acetonitrile and added dropwise to an acetonitrile solution of ornithine, to react and generate a complex; 10 ml 20% sodium bicarbonate aqueous solution was added, and 2 g of anhydrous sodium carbonate and an appropriate amount of 8-hydroxyquinoline were added in batches; the mixture was stirred and reacted at room temperature for 4 hours to remove copper ions in the complex; then 2 mmol Fmoc-OSu was added to the aforesaid solution, followed by stirring at room temperature for 2 hours; the resultant was recrystallized with an ethyl acetate/petroleum ether mixed solvent, to obtain a peptide head intermediate Fmoc-L-Orn (Boc)-OH.
References:
US2017/355667,2017,A1 Location in patent:Paragraph 0059; 0069; 0073; 0085
![L-Ornithine, N5-[(1,1-dimethylethoxy)carbonyl]-N2-[(9H-fluoren-9-ylmethoxy)carbonyl]-, 1,1-dimethyl-2-propen-1-yl ester](/CAS/20210305/GIF/851713-95-6.gif)
851713-95-6
0 suppliers
inquiry

109425-55-0
311 suppliers
$8.00/1g

82911-69-1
605 suppliers
$7.00/25g
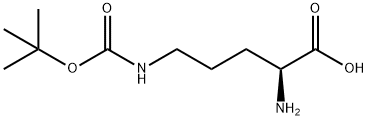
13650-49-2
141 suppliers
$6.00/250mg

109425-55-0
311 suppliers
$8.00/1g

24424-99-5
824 suppliers
$13.50/25G

82911-69-1
605 suppliers
$7.00/25g
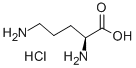
3184-13-2
603 suppliers
$5.00/250mg

109425-55-0
311 suppliers
$8.00/1g

24424-99-5
824 suppliers
$13.50/25G

109425-55-0
311 suppliers
$8.00/1g