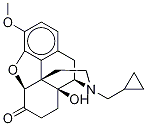
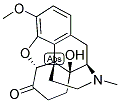
Naltrexone 3-Methyl Ether synthesis
- Product Name:Naltrexone 3-Methyl Ether
- CAS Number:16617-07-5
- Molecular formula:C21H25NO4
- Molecular Weight:355.43
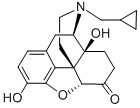
16590-41-3
146 suppliers
$27.89/007-1ml

74-88-4
341 suppliers
$15.00/10g

16617-07-5
26 suppliers
inquiry
Yield: 100%
Reaction Conditions:
with potassium carbonate in dichloromethane for 18 h;
Steps:
10 Alternative methods for Naltrexone Activation
Naltrexone Activation [00364] The ketone of Naltrexone could only be modified after first protecting its phenol as a phenyl methyl ether. The phenol was methylated by dissolving Naltrexone (leq) in 10 ml DCM and then adding potassium carbonate (5eq) followed by iodomethane (20eq). The reaction was stirred for 18 hours and then diluted with DCM and washed several times with a mixture of water and saturated bicarbonate solution followed by drying over magnesium sulfate and concentration under vacuum. The product, O-Me Naltrexone, was recovered in quantitative yield. The ketone of O-Me Naltrexone was then modified by first dissolving potassium bis(trimethylsilyl)amide (KHMDS; 3eq) in 5 ml of dry dimethoxyethylene glycol (DME) in an oven dried flask under Argon and then cooling the mixture to -78°C in a dry ice/acetone bath. O-Me Naltrexone (leq) was added dropwise in 2 ml of dry DME and stirred at -78°C for 30 minutes. Meanwhile, pentafluorophenyl chlorothionoformate (3eq) was dissolved in a separate oven dried flask followed by boron trifluoride etherate (3eq). The solution was then cooled to -78°C in a dry ice/acetone bath for 15 minutes. Next, the solution containing O-Me Naltrexone was cannulated into the other flask and the entire mixture was allowed to stir at -78°C for 30 minutes and then for 60 minutes after removal from the bath. The reaction was then concentrated under vacuum and redissolved in a minimal amount of DCM and precipitated three times in hexanes. Finally, the desired product was recovered by dissolving the remaining material in approximately 60% acetonitrile and purifying it by HPLC using a gradient of 40/60% to 5/95% EhO/acetonitrile gradient with 0.1% TFA over 15 minutes followed by a 5-minute isocratic hold at 95% acetonitrile with 0.1% TFA. Typical yields were around 11% and so the reaction needs to be scaled. The identities of the desired products were confirmed using l NMR, 13C NMR, and ESI-MS.
References:
THE REGENTS OF THE UNIVERSITY OF CALIFORNIA;MAYNARD, Heather, D.;PALUCK, Samantha, J.;BOEHNKE, Natalie WO2018/118903, 2018, A1 Location in patent:Paragraph 00362-00364
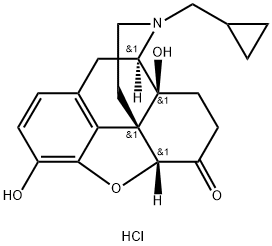
16676-29-2
270 suppliers
$19.00/1mg

74-88-4
341 suppliers
$15.00/10g

16617-07-5
26 suppliers
inquiry

16590-41-3
146 suppliers
$27.89/007-1ml
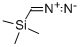
18107-18-1
208 suppliers
$33.00/5g

16617-07-5
26 suppliers
inquiry
76-42-6
2 suppliers
$16.00/1mg

16617-07-5
26 suppliers
inquiry

