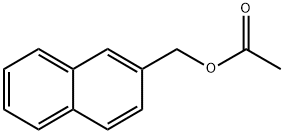
naphthalen-2-ylmethyl acetate synthesis
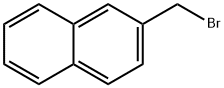
- Product Name:naphthalen-2-ylmethyl acetate
- CAS Number:35480-23-0
- Molecular formula:C13H12O2
- Molecular Weight:200.24
Yield:35480-23-0 99%
Reaction Conditions:
with CH3O3S(1-)*C27H53N2O12(1+) in acetonitrile at 90; for 0.5 h;
Steps:
4.23. General procedure for SN2 reaction
General procedure: MNu (M=KOAc, KSAc, NaN3 or KCN) (3.0 mmol) was added to a mixture of each mesylate, triflate or bromide compound (1.0 mmol), [dihexaEGim][OMs] (346 mg, 0.5 mmol) and acetonitrile (4 mL) in a reaction vial. The reaction mixture was stirred over 0.5-3 h at 90 °C. The reaction mixture was filtered and washed with diethyl ether (15 mL). The filtrate was evaporated under reduced pressure. Flash column chromatography (10% EtOAc/hexanes) of the filtrate afforded each product compound.
References:
Jadhav, Vinod H.;Kim, Ju-Young;Chi, Dae Yoon;Lee, Sungyul;Kim, Dong Wook [Tetrahedron,2014,vol. 70,# 2,p. 533 - 542]