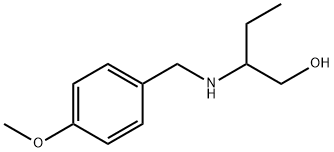
Naproxen Impurity 7 synthesis
- Product Name:Naproxen Impurity 7
- CAS Number:156543-20-3
- Molecular formula:C12H19NO2
- Molecular Weight:209.28
Yield:156543-20-3 99%
Reaction Conditions:
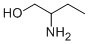
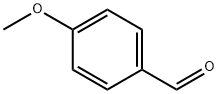
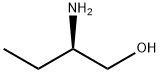
Stage #1: 2-aminobutanol;4-methoxy-benzaldehydewith acetic acid in dichloromethane at 20; for 0.5 h;
Stage #2: with sodium cyanoborohydride in dichloromethane at 0 - 20; for 16 h;
Steps:
2.2.i Synthesis of 2-((4-methoxybenzyl) amino) butan-l-ol (2.2a)
To a stirred solution of 2-aminobutan- 1 -ol ( 1.0 g, 11.23 mmol) and 4-methoxy benzaldehyde (1.83 g, 13.48 mmol) in dichloromethane (15 ml) was added AcOH (0.1 ml, catalytic). The reaction mixture was stirred for 30 min at RT. The reaction mixture was cooled to 0°C then NaCNBH3 (1.41 g, 22.47 mmol) added to the reaction mixture. The reaction mixture was slowly warmed to RT and stirred for about 16 h. The reaction mixture was diluted with sat. sodium bicarbonate solution and the product extracted with ethylacetate. The combined organic layer was washed with water and brine solution. The organic layer was dried over sodium sulfate and concentrated under reduced pressure. The crude material obtained was purified using silica gel column chromatography eluted in 1% MeOH in DCM to afford title compound as gummy compound (2.8 g, 99 %). LC-MS: 210 (M+l)+.
References:
WO2014/108820,2014,A1 Location in patent:Page/Page column 30