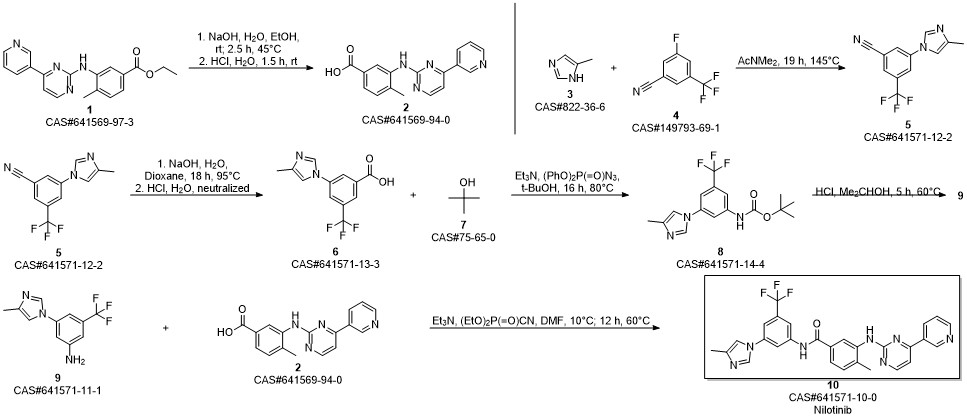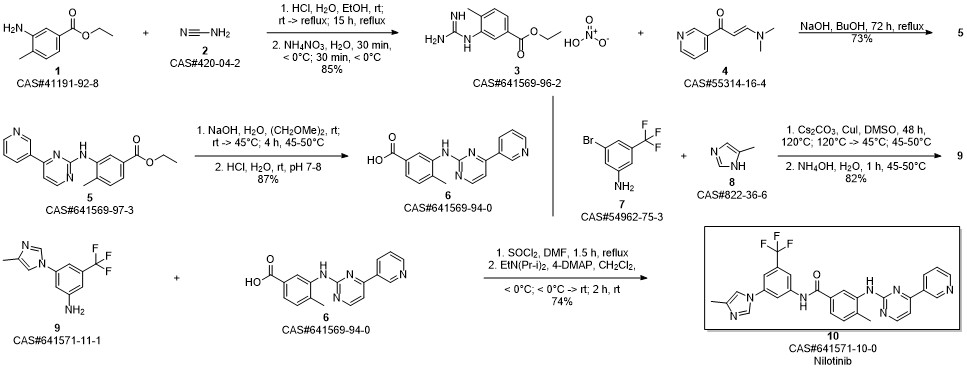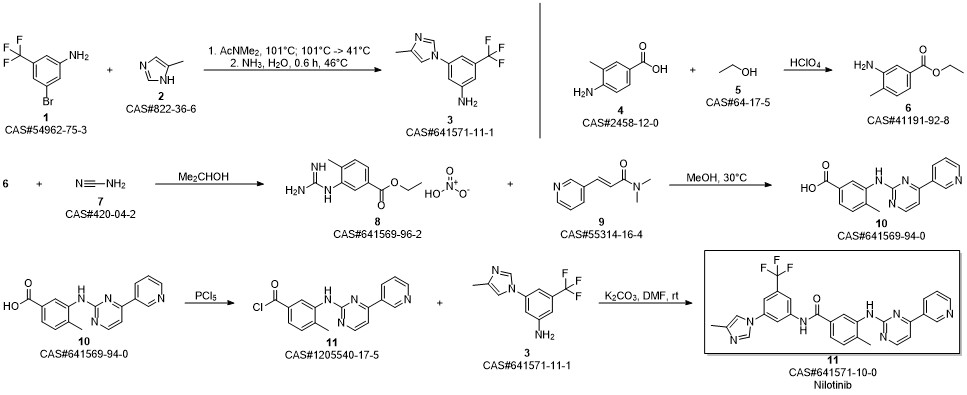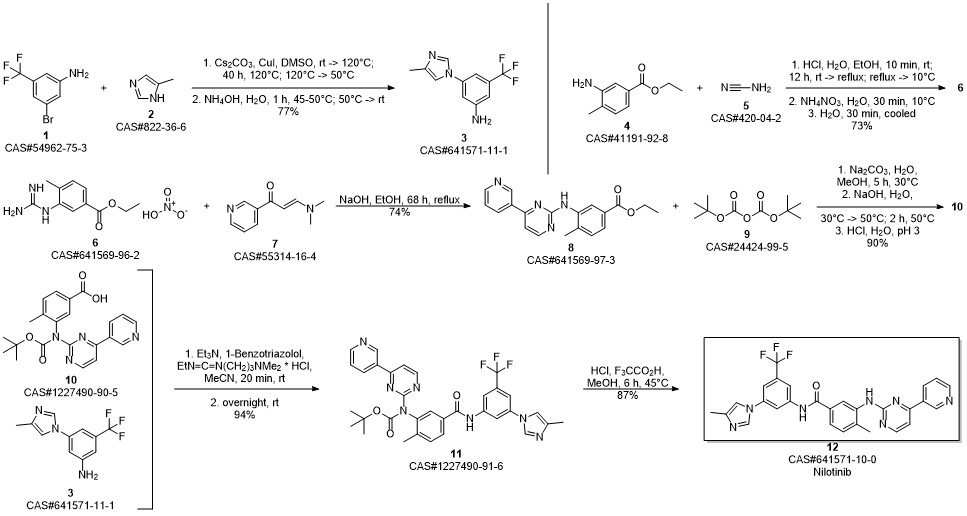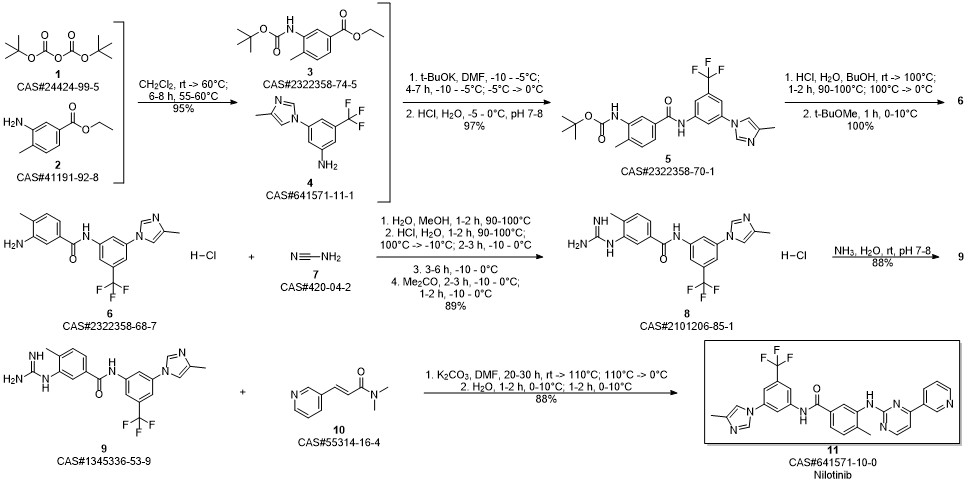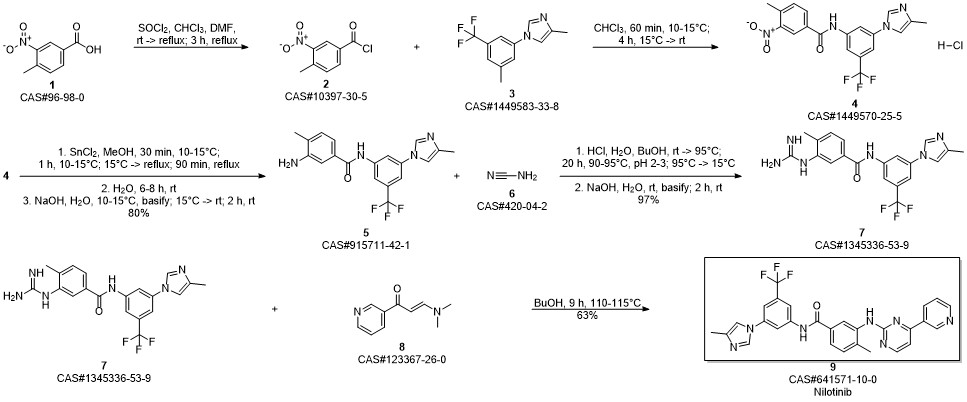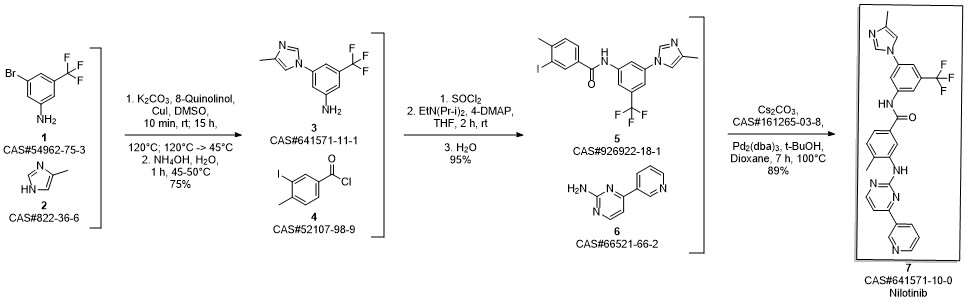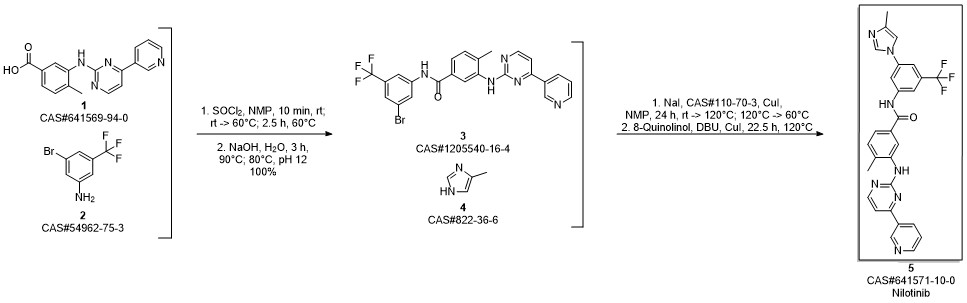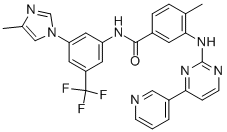
Nilotinib synthesis
- Product Name:Nilotinib
- CAS Number:641571-10-0
- Molecular formula:C28H22F3N7O
- Molecular Weight:529.52
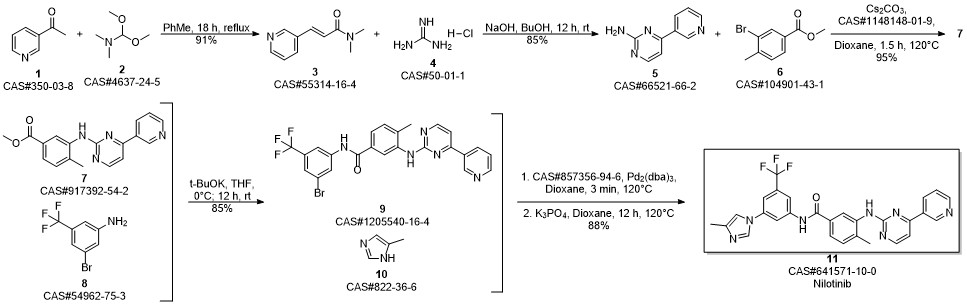
Ueda, Satoshi; Su, Mingjuan; Buchwald, Stephen L. Completely N1-selective palladium-catalyzed arylation of unsymmetric imidazoles: application to the synthesis of nilotinib. Journal of the American Chemical Society. Volume 134. Issue 1. Pages 700-706. 2012.
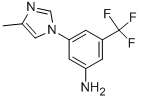
641571-11-1
335 suppliers
$9.00/1g
![4-Methyl-3-[[4-(3-pyridinyl)-2-pyrimidinyl]amino]benzoic acid](/CAS/GIF/641569-94-0.gif)
641569-94-0
240 suppliers
$7.00/250mg

641571-10-0
410 suppliers
$21.00/5mg
Yield:641571-10-0 94%
Reaction Conditions:
Stage #1: 4-methyl-3-((4-(pyridin-3-yl)pyrimidin-2-yl)amino)benzoic acidwith thionyl chloride in 1-methyl-pyrrolidin-2-one at 60; for 1.25 h;
Stage #2: 5-(4-methyl-1H-imidazol-1-yl)-3-(trifluoromethyl)-benzenamine in 1-methyl-pyrrolidin-2-one at 90; for 3 h;Product distribution / selectivity;
Steps:
12
Example 12; Preparation of NilotinibTo 1 L glass reactor was added the compound of formula 4-methyl-3-{[4-(pyridin-3-yl) pyrimidin-2-yl]amino}benzoic acid of formula X (80.0 g, 0.26 mol), and N-Methyl-pyrrolidone (400 mL). The mixture was heated to 60° C., then SOCl2 (24 mL, 0.33 mol) was added during 15 minutes. The resulted mixture was stirred at 60° C. for 1 h. A compound of formula I (69.2 g, 0.29 mol) was added and the reaction mixture was stirred and heated to 90° C. for 3 h. Water (500 mL) was added and the solution was heated to 80° C. NaOH 47% solution (65 mL) was added until pH 11-12. Then, the suspension was cooled to 40° C. and stirred at this temperature for 2 hours, filtered under reduced pressure at 40° C., and washed with 500 mL H2O to yield a beige solid. This material was slurried in water (1 L) at 40° C. for 1 h, filtered, washed with water (500 mL), and dried under vacuum at 50° C. to obtain 135.25 g of Nilotinib base with 94% yield. (95.8% assay, 99.46% purity).
References:
US2010/16590,2010,A1 Location in patent:Page/Page column 20

641571-11-1
335 suppliers
$9.00/1g
![4-Methyl-3-[[4-(3-pyridinyl)-2-pyrimidinyl]amino]benzoic acid methyl ester](/CAS2/GIF/917392-54-2.gif)
917392-54-2
122 suppliers
inquiry

641571-10-0
410 suppliers
$21.00/5mg

641571-11-1
335 suppliers
$9.00/1g
![Benzoyl chloride, 4-methyl-3-[[4-(3-pyridinyl)-2-pyrimidinyl]amino]-](/CAS/20200401/GIF/1205540-17-5.gif)
1205540-17-5
1 suppliers
inquiry

641571-10-0
410 suppliers
$21.00/5mg
![4-Methyl-3-[[4-(3-pyridinyl)-2-pyrimidinyl]amino]benzoic acid](/CAS/GIF/641569-94-0.gif)
641569-94-0
240 suppliers
$7.00/250mg
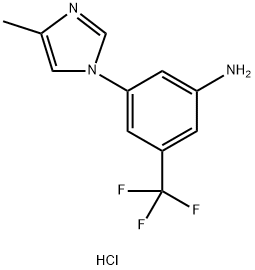
917391-26-5
0 suppliers
inquiry

641571-10-0
410 suppliers
$21.00/5mg
![Benzamide, 3-[(1,6-dihydro-6-oxo-2-pyrimidinyl)amino]-4-methyl-N-[3-(4-methyl-1H-imidazol-1-yl)-5-(trifluoromethyl)phenyl]-](/CAS/20210305/GIF/1451042-83-3.gif)
1451042-83-3
0 suppliers
inquiry
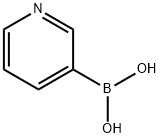
1692-25-7
479 suppliers
$5.00/1g

641571-10-0
410 suppliers
$21.00/5mg