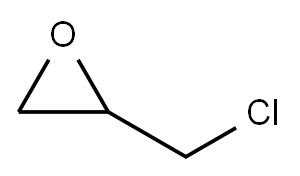
[(nonyloxy)methyl]oxirane synthesis
- Product Name:[(nonyloxy)methyl]oxirane
- CAS Number:10580-65-1
- Molecular formula:C12H24O2
- Molecular Weight:200.32
Yield:10580-65-1 48%
Reaction Conditions:
Stage #1: nonyl alcoholwith sodium hydride in tetrahydrofuran at 0; for 1 h;
Stage #2: 1,2-Epoxy-3-bromopropane in tetrahydrofuran at 20; for 5 h;
Steps:
35-36.A Step A: 2-((nonyloxy)methyl)oxirane.
Nonan-1 -ol (15.0 g, 104 mmol) was dissolved in THF (50 mL ), and the solution treated with sodium hydride (10.4 g, 156 mmol, 60%). After stirring at 0 °C for 1 h, 2-(bromomethyl)oxirane (5.22 g, 38.1 mmol) was added with dropwise. After 5 h at RT, the reaction mixture was diluted with water (100 mL ) and extracted with EtOAc (3x50 mL ). The combined EtOAc extracts were dried over Na2SO4and concentrated at reduced pressure. The crude material was subjected to flash chromatography (silica gel, 10:1 petroleum ether/EtOAc)) to give the desired product (11 .0 g, 48%) as a yellow oil.1H NMR (400 MHz, CDCI3) d 3.70 (dd, J = 11.2, 2.8 Hz, 1 H), 3.54 - 3.33 (m, 3H), 3.16 - 3.09 (m, 1 H), 2.79 (t, J = 4.8 Hz, 1 H), 2.64 - 2.53 (m, 1 H), 1.64 - 1.51 (m, 2H), 1 .34 - 1 .26 (m, 12H), 0.92 - 0.79 (m, 3H).
References:
WO2021/38509,2021,A1 Location in patent:Page/Page column 85

82473-55-0
0 suppliers
inquiry
![[(nonyloxy)methyl]oxirane](/CAS/GIF/10580-65-1.gif)
10580-65-1
2 suppliers
inquiry
693-58-3
215 suppliers
$5.00/5g
![[(nonyloxy)methyl]oxirane](/CAS/GIF/10580-65-1.gif)
10580-65-1
2 suppliers
inquiry