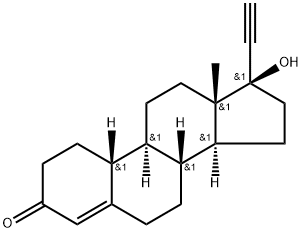
Norethindrone synthesis
- Product Name:Norethindrone
- CAS Number:68-22-4
- Molecular formula:C20H26O2
- Molecular Weight:298.43
Yield:68-22-4 100%
Reaction Conditions:
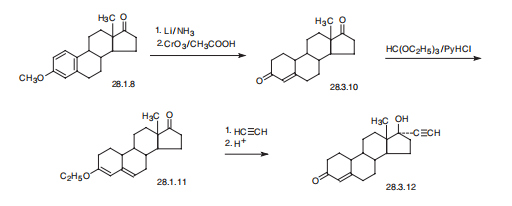
Stage #1:estra-4-ene-3,17-dione with potassium hydroxide in toluene;tert-butyl alcohol under 760.051 Torr; for 0.166667 h;Reflux;Industrial scale;
Stage #2:acetylene in tert-butyl methyl ether;toluene for 6 h;Industrial scale;
Steps:
1.1 Alkylation reaction:
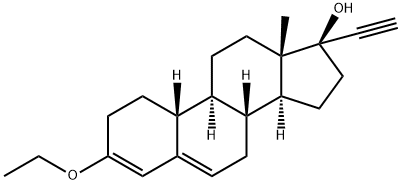
800kg toluene, 20kg potassium hydroxide, 40kg tert-butanol at normal pressure and reflux dehydration for 2 hours, lower the temperature to 30°C, add 100kg acid decarboxylate (I) dissolved in 200kg toluene, and stir for 10 minutes. Acetylene gas was introduced. After 6 hours of ventilation, TLC detection was started. TLC analysis of the raw material reaction was complete. The ventilation was stopped, 200kg of water was added to quench and wash, and the layers were separated. Concentrate under reduced pressure, add 100kg of ethanol, discharge, and dry to obtain 100kg of norethindrone (II), with a mass yield of 100%, detection HPLC ≥99%, single impurity ≤0.5%.
References:
Zhejiang Xianju Junye Pharmaceutical Co., Ltd.;Jiangxi Junye Bio-pharmaceutical Co., Ltd.;Xu Sihu;Zhang Zhengbin;Chen Yinghui;Li Chun;Wu Xiaokai;Pan Baofeng CN110981930, 2020, A Location in patent:Paragraph 0028-0031
96487-85-3
28 suppliers
$130.00/100mg

68-22-4
421 suppliers
$25.00/250mg

734-32-7
209 suppliers
$300.00/1 g

68-22-4
421 suppliers
$25.00/250mg

434-22-0
248 suppliers
$30.00/1mg

68-22-4
421 suppliers
$25.00/250mg

