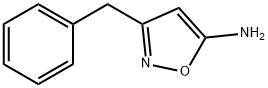
3-(Phenylmethyl)-5-isoxazolamine synthesis
- Product Name:3-(Phenylmethyl)-5-isoxazolamine
- CAS Number:119162-58-2
- Molecular formula:C10H10N2O
- Molecular Weight:174.2
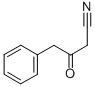
19212-27-2
25 suppliers
inquiry

119162-58-2
10 suppliers
inquiry
Yield:119162-58-2 93%
Reaction Conditions:
with pyridine;hydroxylamine hydrochloride in ethanol at 20; for 84 h;
Steps:
AA.2
Step 2: Synthesis of 3-benzyl-l,2-oxazol-5-amineTo a solution of 3-oxo-4-phenylbutanenitrile (690 mg, 4.34 mmol) in ethanol (25 mL) is added pyridine (4.91 mL, 60.7 mmol) and hydroxylamine hydrochloride (301 mg, 4.34 mmol). The reaction mixture is allowed to stir at room temperature 84 h. The mixture is diluted with ethyl acetate and 1M aqueous NaOH solution. The layers are separated and the aqueous layer is extracted with ethyl acetate. The organic layers are washed with brine, dried (Na2S04), and the solvent is removed in vacuo to afford the title compound as a yellow solid (702 mg, 93%). LCMS: 175.20 (M+H+).
References:
WO2011/71725,2011,A1 Location in patent:Page/Page column 80

101-41-7
493 suppliers
$16.80/100ML

119162-58-2
10 suppliers
inquiry
![Benzeneethanamine, α-methylene-N,N-bis[(trimethylsilyl)oxy]-](/CAS/20210305/GIF/102588-26-1.gif)