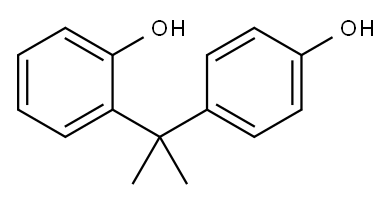
o-[1-(4-hydroxyphenyl)-1-methylethyl]phenol synthesis
- Product Name:o-[1-(4-hydroxyphenyl)-1-methylethyl]phenol
- CAS Number:837-08-1
- Molecular formula:C15H16O2
- Molecular Weight:228.2863
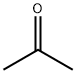
67-64-1
6 suppliers
$17.30/10ml
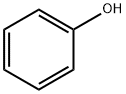
108-95-2
726 suppliers
$14.00/25g
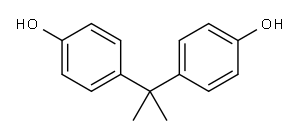
80-05-7
656 suppliers
$13.00/100g
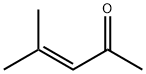
141-79-7
254 suppliers
$10.00/5g
![o-[1-(4-hydroxyphenyl)-1-methylethyl]phenol](/CAS/GIF/837-08-1.gif)
837-08-1
32 suppliers
$185.00/25mg
Yield: 63.2% , 11.43% , 0.01%
Reaction Conditions:
beta zeolite acidic form at 120; under 760.051 Torr; for 12 h;
Steps:
2 EXAMPLE 2-SYNTHESIS OF BISPHENOL A WITH BETA ZEOLITE Bath test at atmospheric pressure
EXAMPLE 2-SYNTHESIS OF BISPHENOL A WITH BETA ZEOLITE Bath test at atmospheric pressure 9.4 g of phenol (0.1 moles) and 1.16 g of acetone (0.02 moles) with a phenol/acetone molar ratio of 5/1, and 1 g of beta zeolite prepared according to example 1, are charged into a glass flask, equipped with a stirrer and water cooler, immersed in a thermostat-regulated oil bath. The above suspension is maintained under stirring for 12 hours, at atmospheric pressure (1 atm), with a bath temperature of 120C. At the end the mass is cooled down to room tempera- ture. The reaction product is analyzed by means of HPLC, with the analysis method described in Journal fur Prak- tische Chemie, Band 328, Heft 1,1986, pp. 142-148. Acetone conversion: 99. 9% Selectivity to p, p'bisphenol A: 63. 20% Selectivity to o, p'bisphenol A: 16. 54% Selectivity to trimers and heavy products: 20.14% Selectivity to mesityl oxide (undesired by-product) lower than the analytical limit (0.01%). EXAMPLE 3-SYNTHESIS OF BISPHENOL A WITH BETA ZEOLITE Bath test, at atmospheric pressure with extraction in continuous of the water formed 9.4 g of phenol (0. 1 moles) and 1.16 g of acetone (0.02 moles) with a phenol/acetone molar ratio of 5/1, and 1 g of beta zeolite prepared according to example 1, are charged into a glass flask, equipped with a stirrer and Dean-Stark trap with a water cooler, immersed in a thermostat-regulated oil bath. The suspension described above is maintained under stirring for 6 hours with a bath temperature of 120C. The system is at atmospheric pressure (1 atmosphere). The toluene-water azeotropic mixture formed during the reaction is continuously removed by means of the Dean- Stark trap. Said azeotropic mixture demixes at room tem- perature: the aqueous phase is removed, whereas toluene is re-fed to the flask. At the end the mass is cooled down to room tempera- ture. The reaction product is analyzed by means of HPLC, with the analysis method described in Journal fur Prak- tische Chemie, Band 328, Heft 1,1986, pp. 142-148. Acetone conversion: 99. 9% Selectivity to p, p'bisphenol A: 72. 31% Selectivity to o, p'bisphenol A: 11. 43% Selectivity to trimers and heavy products: 16.16% Selectivity to mesityl oxide (undesired by-product) lower than the analytical limit (0. 01%).
References:
ENITECNOLOGIE S.P.A. WO2004/48302, 2004, A1 Location in patent:Page 21-22

67-64-1
6 suppliers
$17.30/10ml

108-95-2
726 suppliers
$14.00/25g

80-05-7
656 suppliers
$13.00/100g
![o-[1-(4-hydroxyphenyl)-1-methylethyl]phenol](/CAS/GIF/837-08-1.gif)
837-08-1
32 suppliers
$185.00/25mg
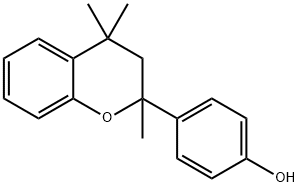
63661-69-8
1 suppliers
inquiry

108-95-2
726 suppliers
$14.00/25g

80-05-7
656 suppliers
$13.00/100g
![o-[1-(4-hydroxyphenyl)-1-methylethyl]phenol](/CAS/GIF/837-08-1.gif)
837-08-1
32 suppliers
$185.00/25mg
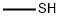
74-93-1
122 suppliers
$454.02/5MG

67-64-1
6 suppliers
$17.30/10ml

108-95-2
726 suppliers
$14.00/25g

80-05-7
656 suppliers
$13.00/100g
![Phenol, 4-[3,4-dihydro-6-[1-(4-hydroxyphenyl)-1-methylethyl]-2,2,4-trimethyl-2H-1-benzopyran-4-yl]-](/CAS/20210111/GIF/287110-79-6.gif)
287110-79-6
1 suppliers
inquiry
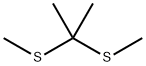
6156-18-9
11 suppliers
inquiry
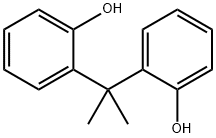
7559-72-0
12 suppliers
$145.00/100mg
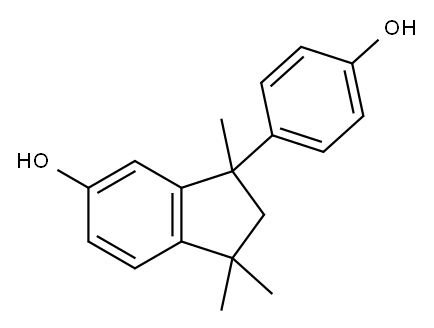
10527-11-4
39 suppliers
inquiry
![2,4-bis[1-(4-hydroxyphenyl)isopropyl]phenol](/CAS/GIF/2300-15-4.gif)
2300-15-4
11 suppliers
inquiry
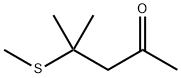
23550-40-5
168 suppliers
$48.00/25G
![o-[1-(4-hydroxyphenyl)-1-methylethyl]phenol](/CAS/GIF/837-08-1.gif)
837-08-1
32 suppliers
$185.00/25mg
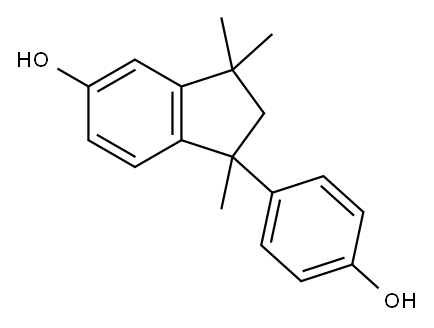
109252-41-7
2 suppliers
inquiry

6156-18-9
11 suppliers
inquiry

67-64-1
6 suppliers
$17.30/10ml

108-95-2
726 suppliers
$14.00/25g

80-05-7
656 suppliers
$13.00/100g
![Phenol, 4-[3,4-dihydro-6-[1-(4-hydroxyphenyl)-1-methylethyl]-2,2,4-trimethyl-2H-1-benzopyran-4-yl]-](/CAS/20210111/GIF/287110-79-6.gif)
287110-79-6
1 suppliers
inquiry
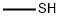
74-93-1
122 suppliers
$454.02/5MG

7559-72-0
12 suppliers
$145.00/100mg

10527-11-4
39 suppliers
inquiry
![2,4-bis[1-(4-hydroxyphenyl)isopropyl]phenol](/CAS/GIF/2300-15-4.gif)
2300-15-4
11 suppliers
inquiry

23550-40-5
168 suppliers
$48.00/25G
![o-[1-(4-hydroxyphenyl)-1-methylethyl]phenol](/CAS/GIF/837-08-1.gif)
837-08-1
32 suppliers
$185.00/25mg

109252-41-7
2 suppliers
inquiry