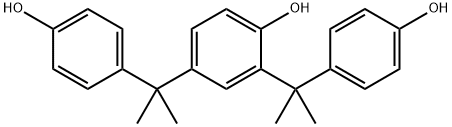
2,4-bis[1-(4-hydroxyphenyl)isopropyl]phenol synthesis
- Product Name:2,4-bis[1-(4-hydroxyphenyl)isopropyl]phenol
- CAS Number:2300-15-4
- Molecular formula:C24H26O3
- Molecular Weight:362.46
74-93-1
119 suppliers
$454.02/5MG

67-64-1
6 suppliers
$17.30/10ml

108-95-2
722 suppliers
$14.00/25g
80-05-7
656 suppliers
$13.00/100g
![Phenol, 4-[3,4-dihydro-6-[1-(4-hydroxyphenyl)-1-methylethyl]-2,2,4-trimethyl-2H-1-benzopyran-4-yl]-](/CAS/20210111/GIF/287110-79-6.gif)
287110-79-6
1 suppliers
inquiry
6156-18-9
10 suppliers
inquiry
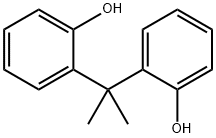
7559-72-0
11 suppliers
$145.00/100mg
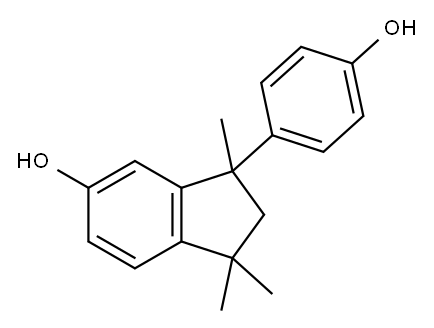
10527-11-4
38 suppliers
inquiry
![2,4-bis[1-(4-hydroxyphenyl)isopropyl]phenol](/CAS/GIF/2300-15-4.gif)
2300-15-4
10 suppliers
inquiry
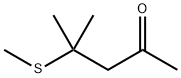
23550-40-5
168 suppliers
$48.00/25G
![o-[1-(4-hydroxyphenyl)-1-methylethyl]phenol](/CAS/GIF/837-08-1.gif)
837-08-1
31 suppliers
$185.00/25mg
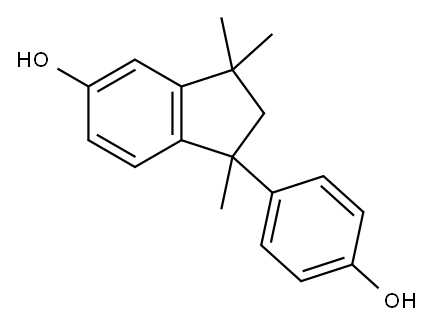
109252-41-7
2 suppliers
inquiry
Yield:-
Reaction Conditions:
strongly acidic 2percent cross linked sulfonated styrene divinylbenzene cationic gel exchange resin CT122 in water at 75; for 3 h;Product distribution / selectivity;
Steps:
1
Rate of reaction for the preparation of BPA; [0056] The rates of the acetone/phenol condensation catalyzed by the strongly acidic cationic exchange resin CT 122, and promoted with MeSH and BMTP, respectively, were measured by in-situ IR. A mixture of phenol, water, and CT122 was preheated to 75°C in an autoclave at autogeneous pressure, followed by injection with a solution of acetone and a promoter. The relative molar composition of the feed in the MeSH-promoted reaction was 100 moles of phenol, 8.1 moles of acetone, 2.8 moles of water, and 1.04 moles of MeSH. The relative molar composition of the feed in the BMTP-promoted reaction was the same except that MeSH was replaced with half the molar amount of BMTP. The weight ratio of phenol to CT 122 was 100 to 3.17 in both reactions. The mixture was reacted over a period of about 3 hours, during which time the reaction rate was measured. The results of this experiment demonstrated that there was no induction period in the BMTP-promoted reaction and its first order rate for conversion of acetone was substantially identical to that of a MeSH-promoted reaction.; Sulfur Species Analysis; [0058] The presence of sulfur by-products in the MeSH and the BMTP promoted reactions were measured by GC-MS of the reaction mixtures. Analysis showed the presence of three sulfur species in each case: methyl mercaptan (MeSH), BMTP and 4-thiomethyl-4-methyl-2-pentanone (TMP). The relative concentrations of the three sulfur species were virtually the same in both the MeSH and BMTP promoted reactions. This is consistent with rapid equilibration of BMTP and MeSH leading to the same distribution of sulfur species under reaction conditions. The small amount of dimethyl disulfide found in the MeSH- promoted reaction was shown to be impurity in MeSH. All numbers were relative areas.
References:
BADGER LICENSING LLC WO2007/130040, 2007, A1 Location in patent:Page/Page column 20-21
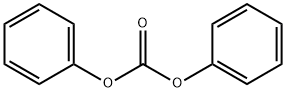
102-09-0
391 suppliers
$6.00/25g
![2,4-bis[1-(4-hydroxyphenyl)isopropyl]phenol](/CAS/GIF/2300-15-4.gif)
2300-15-4
10 suppliers
inquiry

6156-18-9
10 suppliers
inquiry

67-64-1
6 suppliers
$17.30/10ml

108-95-2
722 suppliers
$14.00/25g

80-05-7
656 suppliers
$13.00/100g
![Phenol, 4-[3,4-dihydro-6-[1-(4-hydroxyphenyl)-1-methylethyl]-2,2,4-trimethyl-2H-1-benzopyran-4-yl]-](/CAS/20210111/GIF/287110-79-6.gif)
287110-79-6
1 suppliers
inquiry
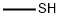
74-93-1
119 suppliers
$454.02/5MG

7559-72-0
11 suppliers
$145.00/100mg

10527-11-4
38 suppliers
inquiry
![2,4-bis[1-(4-hydroxyphenyl)isopropyl]phenol](/CAS/GIF/2300-15-4.gif)
2300-15-4
10 suppliers
inquiry

23550-40-5
168 suppliers
$48.00/25G
![o-[1-(4-hydroxyphenyl)-1-methylethyl]phenol](/CAS/GIF/837-08-1.gif)
837-08-1
31 suppliers
$185.00/25mg

109252-41-7
2 suppliers
inquiry

80-05-7
656 suppliers
$13.00/100g
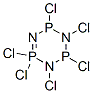
940-71-6
333 suppliers
$20.00/5 g
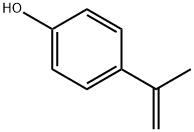
4286-23-1
102 suppliers
$34.00/100mg

10527-11-4
38 suppliers
inquiry
![2,4-bis[1-(4-hydroxyphenyl)isopropyl]phenol](/CAS/GIF/2300-15-4.gif)
2300-15-4
10 suppliers
inquiry
![o-[1-(4-hydroxyphenyl)-1-methylethyl]phenol](/CAS/GIF/837-08-1.gif)
837-08-1
31 suppliers
$185.00/25mg

108-95-2
722 suppliers
$14.00/25g