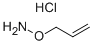
O-ALLYLHYDROXYLAMINE HYDROCHLORIDE synthesis
- Product Name:O-ALLYLHYDROXYLAMINE HYDROCHLORIDE
- CAS Number:38945-21-0
- Molecular formula:C3H8ClNO
- Molecular Weight:109.55
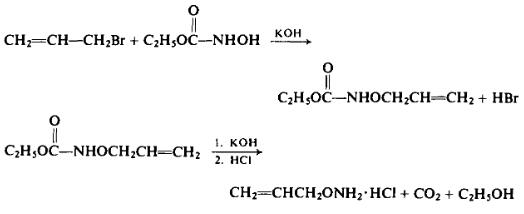
In a steam distillation apparatus, 134 gm of ethyl O-allylhydroxy-carbamate is treated with a solution of 120 gm of potassium hydroxide in 280 ml of water. The product is steam-distilled into a receiver containing dilute hydrochloric acid.
The steam distillate is evaporated under reduced pressure. The residue is taken up twice in absolute ethanol and dried by evaporation under reduced pressure. The yield is 91 gm (55%, overall), m.p. 169-170°C. Upon recrystallization from absolute alcohol and dry ether, the melting point is raised to 170.6-170.8°C (172-174°C). Free O-allylhydrox-ylamine has the following reported properties: b.p. 98-99°C, n25D 1.4300.
The problems connected with the preparation of O-arylhydroxylamines by this method have been attributed to the instability of the aryl- substituted aminooxy group to the hydrolytic system used in its preparation. This problem has recently been circumvented by substituting for ethyl N-hydroxycarbamate, t-butyl N-hydroxycarbamate.
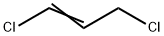
542-75-6
191 suppliers
$11.00/25g

38945-21-0
139 suppliers
$11.00/1g
Yield: 93%
Reaction Conditions:
with N,N,N,N,N,N-hexamethylphosphoric triamide;hydroxylamine hydrochloride;N-benzyl-N,N,N-triethylammonium chloride;sodium hydroxide in N,N-dimethyl-formamide at 30 - 40; for 1 h;Inert atmosphere;Large scale;Solvent;Reagent/catalyst;
Steps:
2 Example 2 to prepare a hydroxylamine salt pot legal O- allyl hydroxylamine implementation
Under nitrogen gas environment, the reactor was put into acetate 810kg (molecular weight 74,11.0kmol), hydroxylamine hydrochloride 500kg (molecular weight 69.5,7.2kmol), 2kgDMF, stirred for 0.5 hour, to maintain 20-30 , dropping concentration of 30 mass% sodium hydroxide solution 1000kg (7.5kmol), after the completion of dropwise addition, the reaction was kept 1 hour;After completion of the reaction, the reaction mixture was added to 2kg benzyl triethyl ammonium chloride and 970 kg of 1,3-dichloropropene (molecular weight 111,8.7kmol), was added dropwise a concentration of 30 mass% sodium hydroxide solution 1000kg (7.5kmol ), and the reaction temperature was controlled at 30-40 deg.] C, the reaction was stirred for 1 hour;To the reaction solution was added at a concentration of 30% by mass of hydrochloric acid 1000kg (8.2kmol), the reaction was warmed to 50-60 , incubated for 3 hours, the reaction was cooled to room temperature, added to a concentration of 30 mass% sodium hydroxide solution and to pH7 -, toluene was added to 500kg, and extracted three times, to the water layer, the toluene solution of hydroxylamine O- allyl chloride to give the product desolventizing 720kg hydroxylamine O- allyl chloride (molecular weight 107.5,6.7kmol), yield 93.0%, content ≥99%.
References:
Yancheng South Chemical Co., Ltd.;Li, You;Luo, Jinchao;Zhang, Yichen;Zhao, Jian CN105601533, 2016, A Location in patent:Paragraph 0027; 0028; 0029; 0030

39020-79-6
68 suppliers
$36.00/5g

38945-21-0
139 suppliers
$11.00/1g
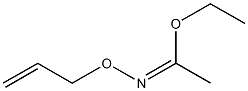
67160-15-0
0 suppliers
inquiry

38945-21-0
139 suppliers
$11.00/1g
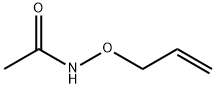
42832-42-8
0 suppliers
inquiry

38945-21-0
139 suppliers
$11.00/1g
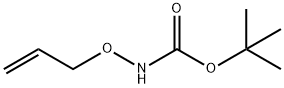
195708-27-1
4 suppliers
inquiry

38945-21-0
139 suppliers
$11.00/1g