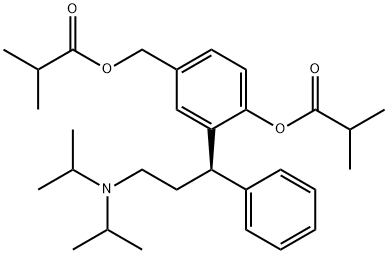
O-Isobutyryl (R)-Fesoterodine synthesis
- Product Name:O-Isobutyryl (R)-Fesoterodine
- CAS Number:1208313-13-6
- Molecular formula:C30H43NO4
- Molecular Weight:481.67
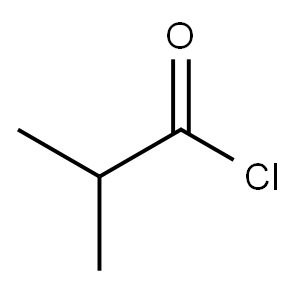
79-30-1
360 suppliers
$10.00/10g
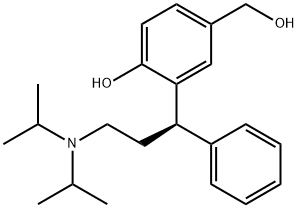
207679-81-0
149 suppliers
$100.00/1g
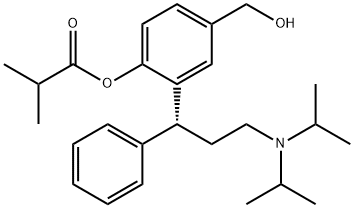
286930-02-7
59 suppliers
inquiry

1208313-13-6
30 suppliers
$499.69/5MG
Yield:-
Reaction Conditions:
with N-ethyl-N,N-diisopropylamine in dichloromethane at -10 - -5; for 2.5 h;Product distribution / selectivity;
Steps:
1; 2
20.0 g of active metabolite is solved in 140 ml dichloromethane (DCM) . The temperature of this solution is adjusted to -100C. Subsequently, a solution of 7.90 g N, iV-diisopropylethylamine (Huenig's base) in 40 ml DCM is added, while the temperature rises to -8°C. The reaction solution is again cooled to -100C, and 6.50 g of isobutyric acid chloride in 120 ml of DCM are added dropwise to the reaction mixture within 30 min, while the temperature is maintained between -8°C and -100C. After stirring for 2 h at a temperature of -100C to -5°C, the reaction mixture was sampled and analysed by HPLC .
References:
WO2007/140986,2007,A1 Location in patent:Page/Page column 12-14