
O-PHENYLHYDROXYLAMINE HYDROCHLORIDE synthesis
- Product Name:O-PHENYLHYDROXYLAMINE HYDROCHLORIDE
- CAS Number:6092-80-4
- Molecular formula:C6H8ClNO
- Molecular Weight:145.59
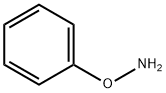
4846-21-3
16 suppliers
$65.00/100mg

6092-80-4
93 suppliers
$45.00/50mg
Yield: 77%
Reaction Conditions:
with hydrogenchloride in 1,4-dioxane;diethyl ether; pH=3 at 0;
Steps:
6; 1A
Representative Procedure for the Hydrazinolysis of N-Aryloxyphthalimides to Corresponding O-Arylhydroxylamines (Method 1A): Synthesis of Compound 1; Hydrazine monohydrate (0.401 mL, 8.2 mmol) was added slowly to a solution of N-phenoxyphthalimide 9 (652 mg, 2.73 mmol) in 10% MeOH in CHCl3 (25 mL) and the reaction was stirred at room temperature. Upon completion (TLC monitoring, 12 h) a white precipitate appeared (the phthalizine) in a colorless reaction solution. The reaction mixture was passed through a plug of silica gel, washing with 30% EtOAc in hexane. Removal of the EtOAc/hexanes produced a slightly pale yellow oil, which upon Kugelrohr distillation from K2CO3 (<10 mg) provided pure phenoxyamine 1 as a clear, colorless oil (238 mg, 80%); see below for characterization data. Alternatively, after removal of the EtOAc/hexanes, the yellow oil was dissolved in Et2O and cooled to 0° C. After 10 min at 0° C., 4 N HCl in dioxane was added dropwise until pH 3 was reached. The resulting white solid was filtered and washed with Et2O (2×10 mL) to afford 1 as the pure HCl salt (306 mg, 77%).; O-Phenylhydroxylamine hydrochloride (1); Preparation of 1 was described above as the representative procedure (method 1A). 1H-NMR (400 MHz, CD3OD) δ 6.84-6.89 (m, 1H), 7.03-7.09 (m, 2H), 7.19-7.25 (m, 2H); 13C-NMR (100 MHz, CD3OD) δ 114.1, 121.6, 130.3, 163.1; LC-MS 110 m/z [MH]+, C6H8NO requires 110.
References:
The Scripps Research Institute US2006/178527, 2006, A1 Location in patent:Page/Page column 8; sheet 6
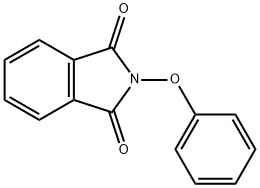
64908-64-1
15 suppliers
inquiry

6092-80-4
93 suppliers
$45.00/50mg
![Ethanamine, N-[(3,5-dichlorophenyl)methylene]-2,2-diethoxy-](/CAS/20210305/GIF/1000210-73-0.gif)
1000210-73-0
0 suppliers
inquiry

6092-80-4
93 suppliers
$45.00/50mg

76570-49-5
3 suppliers
inquiry

6092-80-4
93 suppliers
$45.00/50mg

98-80-6
744 suppliers
$5.00/5g

6092-80-4
93 suppliers
$45.00/50mg