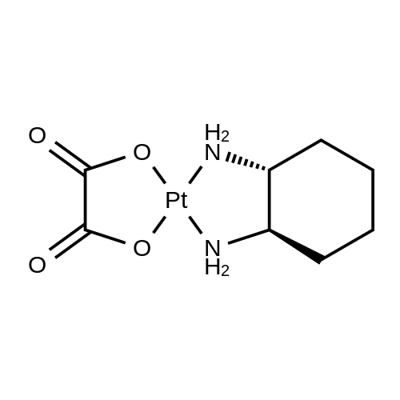
Oxaliplatin synthesis
- Product Name:Oxaliplatin
- CAS Number:61825-94-3
- Molecular formula:C8H12N2O4Pt
- Molecular Weight:395.28

144-62-7
815 suppliers
$5.00/5g

61825-94-3
560 suppliers
$19.00/50mg
Yield:61825-94-3 62.9%
Reaction Conditions:
Stage #1:(1R,2R)-1,2-diaminocyclohexanedichloroplatinum(II) with water;silver nitrate at 20 - 45; for 2.08333 h;
Stage #2:oxalic acid with potassium hydroxide in water; pH=2.9 at 20; for 4 h;Product distribution / selectivity;
Steps:
1; 2; 3
A mixture of 3,88 g of fine powdered DACHPtCI2 98% (10 mmol), 1.55 g purified Celite, 3.41 g AgNO3 99.5% (20 mmol) and 27 ml pu- rified water was intensively stirred 5 minutes at room temperature and then 2 hours at 450C. The suspension was cooled to 30C and then filtered through the plate with active charcoal. The crude acidic filtrate had the content of silver ions 0.0018 mass.% ,i.e. 18 p.p.m. 0.19 g of Smopex-101 was added to the crude filtrate and the sus- pension was stirred 1 hour at room temperature. The solid fraction was then removed by filtration. The purifying procedure with
References:
VUAB PHARMA a.s. WO2007/140804, 2007, A1 Location in patent:Page/Page column 14-16
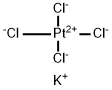
10025-99-7
462 suppliers
$19.00/250mg
20439-47-8
430 suppliers
$5.00/250mg

144-62-7
815 suppliers
$5.00/5g

61825-94-3
560 suppliers
$19.00/50mg
![Platinum, dichloro(1,2-cyclohexanediamine-N,N')-, [sp-4-2-(1R-trans)]-](/CAS/20180713/GIF/61848-66-6.gif)
61848-66-6
55 suppliers
$65.00/1 mg

144-62-7
815 suppliers
$5.00/5g

61825-94-3
560 suppliers
$19.00/50mg
![Platinum, dichloro(1,2-cyclohexanediamine-N,N')-, [sp-4-2-(1R-trans)]-](/CAS/20180713/GIF/61848-66-6.gif)
61848-66-6
55 suppliers
$65.00/1 mg

33081-83-3
3 suppliers
inquiry

61825-94-3
560 suppliers
$19.00/50mg