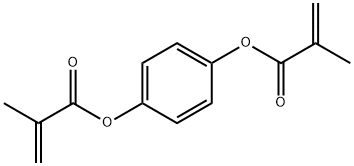
p-hydroxyphenyl methacrylate synthesis
- Product Name:p-hydroxyphenyl methacrylate
- CAS Number:31480-93-0
- Molecular formula:C10H10O3
- Molecular Weight:178.18

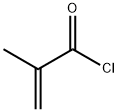
79-41-4
489 suppliers
$14.00/5g
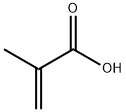
760-93-0
306 suppliers
$28.46/50g
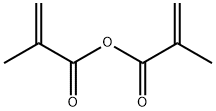
123-31-9
826 suppliers
$13.00/25g
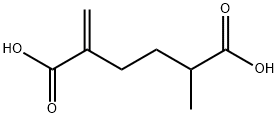
5363-70-2
1 suppliers
inquiry

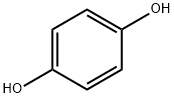
31480-93-0
78 suppliers
inquiry
Yield:31480-93-0 97% ,5363-70-2 1%
Reaction Conditions:
at 120; for 4 h;Product distribution / selectivity;
Steps:
2
Example 2: Monomer synthesis; preparation of hydroxyphenyl methacrylate; To a mechanically stirred solution of 2 moles of hydroxyquinone dissolved in 3 moles of methacrylic acid at 120°C and atmospheric pressure was added dropwise (30 min) 1 mole of methylacrylic acid anhydride and the mixture was maintaned at 120°C with stirring for additional 4 hrs (NMR analysis). Throughout the course of the reaction 8% oxygen was admitted to the system. At the end of the reaction, the methacrylic acid was recovered by distillation under reduced pressure (110°C and 200 mmHg), and the unreacted excess hydroquinone was precipitated out by the addition of toluene (1 liter) to the reaction mixture. A low level (1-2%) of the monomer 2-methyl-5-methylenehexanedioic acid was formede. This monomer was separated from the mixture by a washing step with distilled water. After phase separation, the desired 4-hydroxyphenyl methacrylate was obtained at 97% yield by the distillation of toluene under reduced pressure. {mp. (Uncorrected) 120°C; Anal. Calcd. For C10H10O3: C, 67.41 : H, 5.66 ; O, 26.94 . Found: C, 67.37 ; H, 5,62}.
References:
EP1970367,2008,A2 Location in patent:Page/Page column 6

79-41-4
489 suppliers
$14.00/5g

123-31-9
826 suppliers
$13.00/25g

31480-93-0
78 suppliers
inquiry
3049-31-8
17 suppliers
$130.00/1g