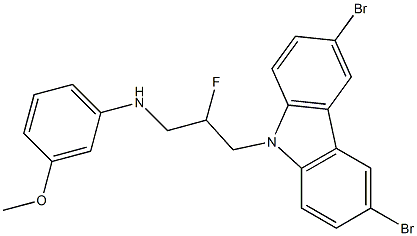
P7C3-A20 synthesis
- Product Name:P7C3-A20
- CAS Number:1235481-90-9
- Molecular formula:C22H19Br2FN2O
- Molecular Weight:506.21
![Ethanamine, N-[(3,5-dichlorophenyl)methylene]-2,2-diethoxy-](/CAS/20210305/GIF/1000210-73-0.gif)
1000210-73-0
0 suppliers
inquiry

1235481-90-9
84 suppliers
$28.00/1mg
Yield: 88%
Reaction Conditions:
with mercaptoacetic acid;lithium hydroxide in N,N-dimethyl-formamide at 20; for 1 h;
Steps:
6a.5 N-(3-(3,6-dibromo-9H-carbazol-9-yl)-2-fluoropropyl)-3-methoxyaniline
To a vial containing N-(3-(3,6-dibromo-9H-carbazol-9-yl)-2-fluoropropyl)-N-(3-methoxyphenyl)-4-nitrobenzenesulfonamide (21.0 mg, 0.030 mmol; see representative procedure 4) was added lithium hydroxide (3.2 mg, 0.134 mmol), dimethylformamide (0.5 ml, 0.06 M) and mercaptoacetic acid (4.2 ul 0.060 mmol).
After stirring at rt for 1 h the reaction mixture was diluted with EtOAc and washed sequentially with water, saturated sodium bicarbonate solution, water (3*) and brine.
The organic layer was dried over Na2SO4, filtered and condensed.
The crude reaction mixture was purified in 30% EtOAc/hexanes (+0.2% TEA), with 13.6 mg isolated. Yield=88% Additional Representative Procedure (1370) DAST [(Et2NSF3) 0.12 ml, 0.916 mmol] was added dropwise to a solution of 1-(3,6-dibromo-9H-carbazol-9-yl)-3-(3-methoxyphenylamino)propan-2-ol (0.102 g, 0.203 mmol) in 6.0 ml of anhydrous DCM at -78° C. The reaction was stirred at -78° C. for one hour before being slowly warmed to 0° C. over 5 hours. The reaction was quenched by addition of phosphate buffer (pH=8) and extracted with DCM. The aqueous phase was extracted twice with 10 ml DCM. The combined organics were dried over Na2SO4, filtered and concentrated. The crude reaction material was purified by flash chromatography on Si02 (20% EtOAc/hexanes/0.2% TEA). Fractions containing the desired fluorinated product were further purified with 40% EtOAc/hexanes (+0.1% TEA). Isolated 5.7 mg desired product. Analytical Data for the Title Compound of Example 6a (1371) 1H NMR (CDCl3, 500 MHz) ζ 8.16 (2H, J=2.0 Hz), 7.56 (dd, 2H, J=1.9, 8.7 Hz), 7.31 (d, 2H, J=8.6 Hz), 7.11 (t, 1H, J=8.1 Hz), 6.36 (dd, 1H, J=2.2, 8.1 Hz), 6.23 (dd, 1H, J=2.0, 8.0 Hz), 6.15 (t, 1H, J=2.3 Hz), 5.11 (dddd, 1H, J=4.6, 5.8, 10.4, 47.7 Hz), 4.60 (m, 2H), 4.39 (dm, 2H), 3.95 (t, 1H, J=6.3 Hz), 3.75 (s, 3H) (1372) MS (ESI), m/z: 504.9 (M+1)+. ([M+1]+ for C22H19Br2FN2O calculated 505.0)
References:
Board of Regents of The University of Texas System;McKnight, Steven L.;Pieper, Andrew A.;Ready, Joseph M.;De Brabander, Jef K. US9095571, 2015, B2 Location in patent:Page/Page column 97; 98

313268-18-7
11 suppliers
inquiry

1235481-90-9
84 suppliers
$28.00/1mg
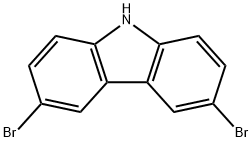
6825-20-3
328 suppliers
$5.00/5g

1235481-90-9
84 suppliers
$28.00/1mg

85446-05-5
28 suppliers
$212.00/500mg

1235481-90-9
84 suppliers
$28.00/1mg
![Ethanamine, N-[(3,5-dichlorophenyl)methylene]-2,2-diethoxy-](/CAS/20210305/GIF/1000210-73-0.gif)
1000210-73-0
0 suppliers
inquiry

1235481-90-9
84 suppliers
$28.00/1mg
![Ethanamine, N-[(3,5-dichlorophenyl)methylene]-2,2-diethoxy-](/CAS/20210305/GIF/1000210-73-0.gif)
1000210-73-0
0 suppliers
inquiry