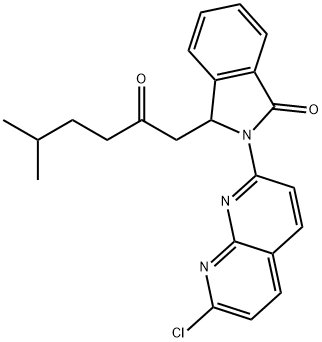
Pagoclone synthesis
- Product Name:Pagoclone
- CAS Number:133737-48-1
- Molecular formula:C23H22ClN3O2
- Molecular Weight:407.89

42809-49-4
2 suppliers
inquiry
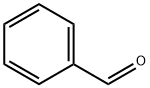
100-52-7
966 suppliers
$20.37/250g

55243-02-2
91 suppliers
$70.00/100mg

133737-48-1
15 suppliers
$50.00/1mg

133737-48-1
15 suppliers
$50.00/1mg
Yield:133737-48-1 15%
Reaction Conditions:
with dichloro(pentamethylcyclopentadienyl)rhodium (III) dimer;copper diacetate in acetonitrile at 80; for 10 h;Schlenk technique;Inert atmosphere;
Steps:
1 Example 1
In a 25mL Schlenk bottle, 22 mg (0.2 mmol) of p-methylbenzaldehyde (1.0 eq), Cu(OAc) 2 65 mg (2.0 eq), 2-amino 1,8-naphthyridine 54 mg (1.5 eq), [Cp*RhCl2] 2 6 mg (5%). Then, it was evacuated and then purged with nitrogen. After three operations, 2 mL of acetonitrile and 50 mg of an unsaturated carbonyl compound (2.0 eq) were added, and the mixture was stirred at 80 ° C under a nitrogen atmosphere. After about 10 hours, the reaction was completed by TLC, a little silica gel was added, and the solvent was distilled off. Curing, solid loading, column chromatography. The total yield of pagoclone (cas: 133737-32-3) can be prepared to be about 15%.
References:
CN109485647,2019,A Location in patent:Paragraph 0029; 0030; 0031; 0032; 0033

42809-49-4
2 suppliers
inquiry

100-52-7
966 suppliers
$20.37/250g

15944-33-9
32 suppliers
$90.00/50mg

133737-48-1
15 suppliers
$50.00/1mg

133737-48-1
15 suppliers
$50.00/1mg

110-12-3
195 suppliers
$10.00/5mL

133737-48-1
15 suppliers
$50.00/1mg

133737-48-1
15 suppliers
$50.00/1mg

141-86-6
505 suppliers
$6.00/5g

133737-48-1
15 suppliers
$50.00/1mg

133737-48-1
15 suppliers
$50.00/1mg

632620-24-7
10 suppliers
inquiry

133737-48-1
15 suppliers
$50.00/1mg

133737-48-1
15 suppliers
$50.00/1mg