Paliperidone Impurity 12 synthesis
- Product Name:Paliperidone Impurity 12
- CAS Number:1117803-76-5
- Molecular formula:C13H17ClN2O3
- Molecular Weight:284.74
![3-(2-Chloroethyl)-2-methyl-9-hydroxy-4H-pyrido[1,2-a]pyrimidin-4-one (Paliperidone)](/CAS/GIF/260273-82-3.gif)
260273-82-3
56 suppliers
inquiry

1117803-76-5
13 suppliers
inquiry
Yield:-
Reaction Conditions:
with hydrogen;acetic anhydride;acetic acid;palladium in water;
Steps:
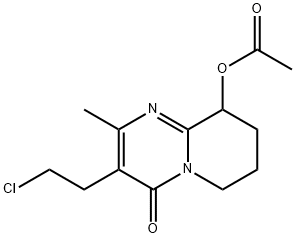
3 Preparation of 3-(2-chloroethyl)-6,7,8,9-tetrahydro-9-acetyloxy-2-methyl-4H-pyrido[1,2-A]pyrimidin-4-one (Formula VIII)
EXAMPLE 3 Preparation of 3-(2-chloroethyl)-6,7,8,9-tetrahydro-9-acetyloxy-2-methyl-4H-pyrido[1,2-A]pyrimidin-4-one (Formula VIII) 3-(2-chloroethyl)-2-methyl-9-hydroxy-4H-pyrido[1,2-a]pyrimidin-4-one (70 g), acetic acid (1400 mL), acetic anhydride (70 mL) and 5% w/w palladium on carbon (35 g) were placed into a clean and dry autoclave vessel. To this reaction mass 6 kg/cm2 hydrogen pressure was applied and the reaction mass was heated to 40° C. The reaction mass was maintained under agitation at 40° C. for 7 hours. After completion of the reaction, the reaction suspension was filtered and the solid washed with acetic acid (70 mL). The solvent was distilled completely from the filtrate at 50° C. under vacuum. To the residue water (700 mL) was added and the mixture was extracted with dichloromethane (3*350 mL). Combined organic layer was washed with water (2*350 mL) and saturated sodium chloride solution (350 mL). Organic layer was dried over anhydrous sodium sulphate and the solvent was distilled completely at 30° C. under vacuum to afford 75 g of the title compound.
References:
US2009/48272,2009,A1