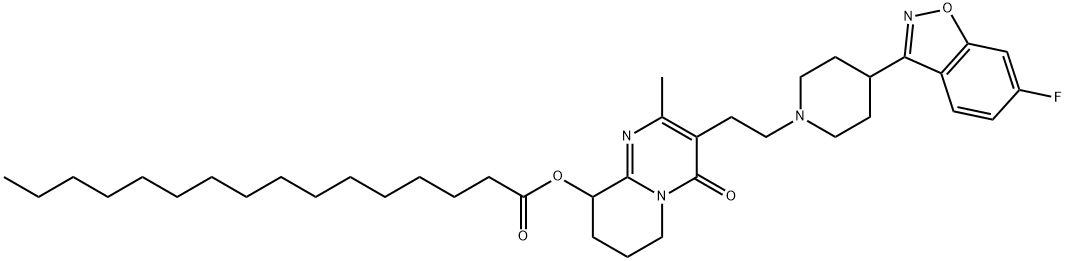
Paliperidone Palmitate synthesis
- Product Name:Paliperidone Palmitate
- CAS Number:199739-10-1
- Molecular formula:C39H57FN4O4
- Molecular Weight:664.89
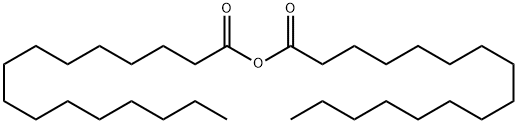
623-65-4
103 suppliers
$20.00/1g

144598-75-4
522 suppliers
$5.00/10mg

199739-10-1
224 suppliers
$70.00/10mg
Yield:199739-10-1 88.5%
Reaction Conditions:
Stage #1: palmitic anhydride;paliperidonewith dmap in toluene at 20 - 70; for 3 h;
Stage #2: with tert-butyl methyl ether in toluene at 10; for 1 h;Product distribution / selectivity;
Steps:
15
A 250 ml reactor equipped with a mechanical stirrer and a reflux condenser was charged under N2 with paliperidone (1O g, 23.45 mmol, 1 eq), palmitic anhydride (14.2 g, 28.7 mmol, 1.2 eq), 4-dimethylaminopyridine (0.72 g, 5.9 mmol, 0.25 eq) and toluene (100 ml). The suspension was heated to 70°C and was stirred for 2 hours, then cooled to 22° +/- 2°C. The suspension was stirred at the same temperature for 1 hour, methyl t-butyl ether (100 ml) was added and the suspension was cooled to 100C and was stirred for 1 hour at the same temperature. The suspension was then filtered under reduced pressure, rinsed with methyl t-butyl ether (40 ml) and dried at 50°C under vacuum. The resulting solid was paliperidone palmitate crude (yield: 29.35 g, purity: 99.7% area by HPLC, compounds related to palmitic acid were less then 0.15%). Yield: 88.5% by wt.
References:
WO2009/89076,2009,A2 Location in patent:Page/Page column 15; 29
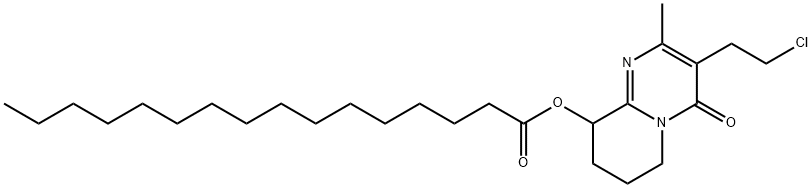
1415488-05-9
18 suppliers
inquiry
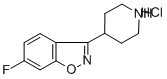
84163-13-3
386 suppliers
$10.00/1g

199739-10-1
224 suppliers
$70.00/10mg

57-10-3
559 suppliers
$5.00/250mg

144598-75-4
522 suppliers
$5.00/10mg

199739-10-1
224 suppliers
$70.00/10mg

144598-75-4
522 suppliers
$5.00/10mg
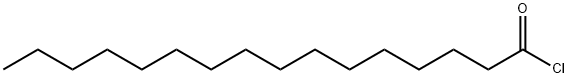
112-67-4
264 suppliers
$10.00/5mL

199739-10-1
224 suppliers
$70.00/10mg
![4H-Pyrido[1,2-a]pyrimidin-4-one, 3-[2-[4-(6-fluoro-1,2-benzisoxazol-3-yl)-1-piperidinyl]ethyl]-6,7,8,9-tetrahydro-9-hydroxy-2-methyl-, sodium salt (1:1)](/CAS/20211123/GIF/1172995-09-3.gif)
1172995-09-3
0 suppliers
inquiry

112-67-4
264 suppliers
$10.00/5mL

199739-10-1
224 suppliers
$70.00/10mg