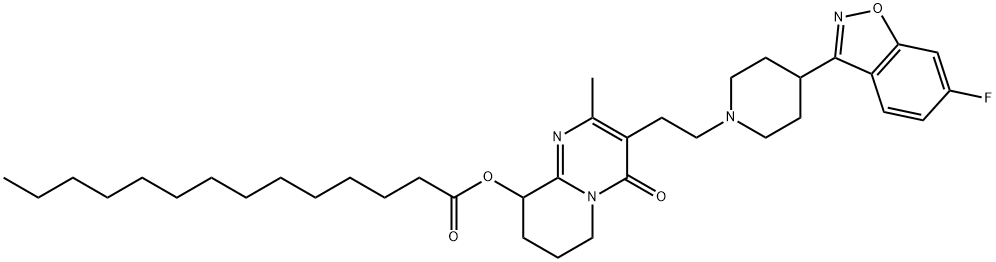
RSHHPAGGAYYRTP-UHFFFAOYSA-N synthesis
- Product Name:RSHHPAGGAYYRTP-UHFFFAOYSA-N
- CAS Number:1172995-11-7
- Molecular formula:C37H53FN4O4
- Molecular Weight:636.84
544-63-8
586 suppliers
$5.00/5g

144598-75-4
513 suppliers
$5.00/10mg

1172995-11-7
20 suppliers
inquiry
Yield:1172995-11-7 95%
Reaction Conditions:
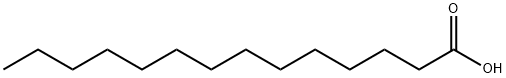
Stage #1: n-tetradecanoic acid;paliperidonewith dmap;benzoyl chloride;triethylamine in tetrahydrofuran at 20; for 6 h;
Stage #2: with water in tetrahydrofuran at 20; for 1 h;
Steps:
18
A 100 ml flask equipped with a magnetic stirrer was charged with paliperidone (5 g, 11.7 mmol, 1 eq), myristic acid (2.67 g, 11.7 mmol, 1 eq), 4-dimethylaminopyridine (0.36 g, 2.95 mmol, 0.25 eq), triethylamine (3.25 ml, 23.3 mmol, 2 eq) and tetrahydrofuran (50 ml). The suspension was stirred at room temperature for 15 minutes. Benzoyl chloride (1.55 ml, 13.34 mmol, 1.1 eq) was added drop-wise and the suspension was further stirred at room temperature for 6 hours. Water (50 ml) was then added and the suspension was further stirred for 1 hour then filtered off under vacuum, washed with water and dried at 500C under vacuum. The resulting solid was paliperidone myristate (purity: 97.5% area by HPLC, yield: 6.25 g, 95%).
References:
WO2009/89076,2009,A2 Location in patent:Page/Page column 30