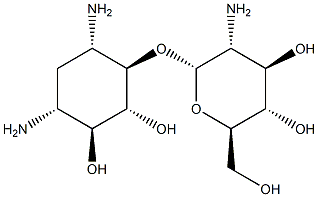
paromamine synthesis
- Product Name:paromamine
- CAS Number:534-47-4
- Molecular formula:C12H25N3O7
- Molecular Weight:323.34
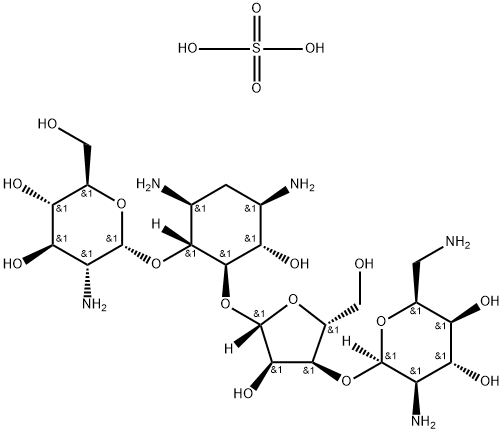
7205-49-4
2 suppliers
inquiry

534-47-4
16 suppliers
inquiry
Yield:534-47-4 94%
Reaction Conditions:
with methanol;acetyl chloride at 0 - 70; for 4.41667 h;
Steps:
1
Preparation ofparomamine (Compound 1):Compound 1Compound 1 was prepared by direct Lewis acid promoted cleavage of paromomycin according to the published procedure of Swayze and co-workers [74] with some modifications.Acetyl chloride (35 ml) was added to a stirred solution of anhydrous methanol (215 ml) over 10 minutes at 0 °C. After stirring for about 15 additional minutes, a commercially available paromomycin sulfate sample (25 grams, 31.0 mmol) was added and the reaction was heated to 70 °C under reflux. Propagation of the reaction was monitored by TLC, using a mixture of CH2Cl2/MeOH/H2O/MeNH2 at a relative ratio of 10:15:6:15 diluted to 33 % solution in ethanol as eluent, which indicated completion after 4 hours. The reaction mixture was cooled for about 2 hours in a freezer, filtered, and the residue was dissolved in a minimal amount of water. This concentrated aqueous solution was added dropwise to cold ethanol (500 ml, 0 0C) and the resulting emulsion was placed in a freezer for about 2 hours. The mixture was filtered and the residue was dried under vacuum to yield Compound 1 as a white solid (12.5 g, 94 % yield).1H NMR (500 MHz, D2O, pH=3.5) data of Compound 1 are summarized in the Table 1 below.
References:
WO2007/113841,2007,A2 Location in patent:Page/Page column 58-59
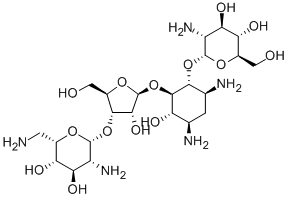
7542-37-2
32 suppliers
inquiry

534-47-4
16 suppliers
inquiry
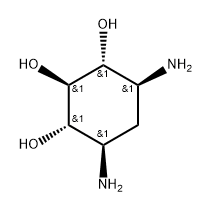
2037-48-1
39 suppliers
inquiry

534-47-4
16 suppliers
inquiry
166516-67-2
0 suppliers
inquiry

534-47-4
16 suppliers
inquiry

236115-65-4
1 suppliers
inquiry

534-47-4
16 suppliers
inquiry