
PEG9-Tos synthesis
- Product Name:PEG9-Tos
- CAS Number:886469-23-4
- Molecular formula:C23H40O11S
- Molecular Weight:524.62
![Bis[2-(2-hydroxyethoxy)ethyl] ether](/CAS/GIF/112-60-7.gif)
112-60-7
347 suppliers
$7.00/25g

98-59-9
585 suppliers
$9.00/5g

886469-23-4
38 suppliers
$198.00/100mg
Yield:886469-23-4 89%
Reaction Conditions:
Stage #1: Tetraethylene glycolwith N,N-dimethyl-4-aminopyridine;[chloro(diphenyl)methyl]benzene;triethylamine in toluene at 20 - 120; for 3 h;Inert atmosphere;
Stage #2: with methanesulfonyl chloride;triethylamine in toluene at 10 - 20; for 2 h;Inert atmosphere;
Stage #3: Tetraethylene glycol;4-methylbenzene-1-sulfonyl chlorideFurther stages;
Steps:
1-4
Tetraethylene glycol 1 (3416 g, 17.6 mol) and toluene (3.7 L) were added to a reactor fitted with a thermometer, a nitrogen inlet tube and a stirrer and the whole was dissolved under a nitrogen atmosphere, followed by azeotropic dehydration at 110 to 120° C. After the azeotropic dehydration, the mixture was cooled, triethylamine (736 ml, 5.3 mol), DMAP (54 g, 0.44 mol) and trityl chloride (TrtCl, 1226 g, 4.4 mol) were added, and the mixture was stirred at room temperature for 3 hours. After 3 hours, the disappearance of TrtCl was confirmed using thin layer chromatography (TLC), a 5% aqueous sodium dihydrogen phosphate solution (6.1 L) was added, and liquid separation was performed. The organic layer was washed once with a 5% aqueous sodium dihydrogen phosphate solution (6.1 L), once with a saturated aqueous sodium hydrogen carbonate solution (6.1 L), and once with a saturated aqueous sodium chloride solution (6.1 L). Sodium sulfate was added to the organic layer for dehydration, followed by filtration. The filtrate was concentrated under reduced pressure to obtain a reaction product containing the compound 2 as a pale yellow transparent liquid. Moreover, it was confirmed from TLC analysis and 1H-NMR measurement results that the obtained reaction product contained the above compound 3.Compound 21H-NMR (CDCl3, internal standard TMS); δ (ppm):2.4 (1H, t, -C-(OCH2CH2)4-OH),3.23 (2H, t, (C6H5)3C-OCH2CH2-, including one derived from compound 3)3.45-3.85 (14H, m, -OCH2CH2-(OCH2CH2)3-OH, including one derived from compound 3),7.21-7.47 (15H, m, (C6H5)3C-OCH2CH2-, including one derived from compound 3)Yield: 1687 g; The reaction product containing the compound 2 (compound 2: 1672 g, less than 3.83 mol) and toluene (8.4 L) were added to a reaction vessel fitted with a thermometer, a nitrogen inlet tube and a stirrer and the product was dissolved under a nitrogen atmosphere, followed by addition of triethylamine (643 ml, 4.62 mol). Methanesulfonyl chloride (326 mL, 4.22 mol) was added dropwise at 10° C., and the mixture was stirred at room temperature for 2 hours. After 2 hours, the disappearance of the compound 2 was confirmed by TLC analysis, a 5% aqueous sodium dihydrogen phosphate solution (8.4 L) was added, and liquid separation was performed. The organic layer was washed once with a 5% aqueous sodium dihydrogen phosphate solution (8.4 L), once with a saturated aqueous sodium hydrogen carbonate solution (8.4 L), and once with a saturated aqueous sodium chloride solution (8.4 L). Sodium sulfate was added to the organic layer for dehydration, followed by filtration. The filtrate was concentrated under reduced pressure to obtain a reaction product containing the compound 4 as a pale yellow transparent liquid. Moreover, it was confirmed from TLC analysis and 1H-NMR measurement results that the obtained reaction product contained the above compound 3.Compound 41H-NMR (CDCl3, internal standard TMS); δ (ppm):2.98 (3H, s, -OCH2CH2-O-SO2CH3),3.23 (2H, t, (C6H5)3C-OCH2CH2-, including one derived from compound 3)3.45-3.85 (12H, m, -OCH2CH2-(OCH2CH2)2-OCH2CH2-, including one derived from compound 3),4.33 (2H, t, -OCH2CH2-O-SO2CH3),7.21-7.47 (15H, m, (C6H5)3C-OCH2CH2-, including one derived from compound 3)Yield: 1942 g From the 1H-NMR measurement results of the compound 4 of Example 1-1, it was confirmed that the compound 3 was contained in an amount of about 6.2 mol %.A calculation expression of the compound 3 content on the basis of a δ 3.23 peak is expressed by the following expression. (((2-[δ 4.32])/4H)/([δ 4.32]/2H))×100 (mol %) Further, the reaction product 2 obtained in Example 1-1 contains the compound 3 in an amount of about 8.8 wt %.; Sodium hydride (215 g) was placed in a reactor fitted with a thermometer, a nitrogen inlet tube and a stirrer, and after nitrogen substitution, MeCN (3.9 L) was added and the mixture was cooled to 0° C. MeCN (2.1 L) was mixed with tetraethylene glycol 1 (3660 g, 18.8 mol) azeotropically dehydrated with toluene (1.8 L), and this mixed solution was added dropwise over 2 hours. After completion of the dropwise addition, MeCN (2.1 L) was mixed with the reaction product containing the compound 4 (compound 4: 1942 g, less than 3.77 mol), and the mixed solution was added dropwise over 20 minutes. After completion of the dropwise addition, the reaction mixture was heated to 75° C. and stirred for 3 hours. After 3 hours, it was confirmed using TLC that the compound 4 had disappeared, and the mixture was allowed to cool until the temperature became 40° C. or lower. A saturated aqueous ammonium chloride solution (3.9 L) and hexane (3 L) were added to the reaction mixture solution and liquid separation was performed. The lower layer from which the hexane layer (upper layer) had been removed was concentrated under reduced pressure, and toluene (9.7 L) was added to the residue. This toluene solution was washed once with a saturated aqueous ammonium chloride solution (5.8 L) and three times with a saturated aqueous sodium chloride solution (9.7 L). Sodium sulfate was added to the organic layer for dehydration, followed by filtration. The filtrate was concentrated under reduced pressure to obtain a reaction product containing the compound 5 as a pale yellow transparent liquid. Moreover, it was confirmed from TLC analysis and 1H-NMR measurement results that the obtained reaction products contained the above compounds 3 and 6.Compound 41H-NMR (CDCl3, internal standard TMS); δ (ppm):2.52 (1H, t, -C-(OCH2CH2)8-OH),3.23 (2H, t, (C6H5)3C-OCH2CH2-, including those derived from compounds 3 and 6)3.45-3.85 (30H, m, -OCH2CH2-(OCH2CH2)7-OH, including those derived from compounds 3 and 6),7.21-7.47 (15H, m, (C6H5)3C-OCH2CH2-, including those derived from compounds 3 and 6)Yield: 2195 g; After the reaction product containing the compound 5 (compound 5: 5 g, less than 8.2 mmol) and dichloromethane (25 mL) was added to a reaction vessel fitted with a thermometer, a nitrogen inlet tube and a stirrer, and the product was dissolved under a nitrogen atmosphere, triethylamine (1.2 mL, 8.6 mmol), 4-dimethylaminopyridine (100 mg, 0.82 mmol) and TsCl (1.4 g, 7.3 mmol) were added, and the mixture was stirred at room temperature for 4 hours. After 4 hours, the disappearance of TsCl was confirmed by 1H-NMR analysis, a 1M aqueous hydrochloric acid solution (25 mL) was ; The reaction product containing the compound 41 (compound 41: 85.6 g, less than 0.144 mol), methanol (20 mL), palladium carbon (Pd/C, 2 g) were added to a reaction vessel fitted with a thermometer, a nitrogen inlet tube and a stirrer, hydrogen substitution was performed, and the mixture was stirred at room temperature for 18 hours. After 18 hours, the disappearance of the compound 42 was confirmed by TLC, and Pd/C was removed by celite filtration. Ion-exchanged water (130 mL) was added to the filtrate, and formed triphenylmethane was filtered. Since triphenylmethane remained in the filtrate, it was washed with hexane (100 mL) five times to remove triphenylmethane. The methanol/ion-exchanged water layer was concentrated under reduced pressure to obtain a crude product containing the compound 41. Then, dichloromethane (120 mL) was added to the crude product and the mixture was washed three times with ion-exchanged water (100 mL) and twice with a saturated aqueous sodium chloride solution (100 mL) under the condition of 20° C. Sodium sulfate was added to the organic layer, which was dried and filtered. The filtrate was concentrated under reduced pressure to obtain a purified product of the compound 42 as a pale yellow transparent liquid.Compound 42Purified product1H-NMR (CDCl3, internal standard TMS); δ (ppm):2.45 (3H, s, -OSO2-Ph-CH3),2.73 (1H, t, H-(OCH2CH2)8-),3.45-3.85 (30H, m, -(OCH2CH2)7-OCH2CH2-),4.16 (2H, t, -(OCH2CH2)7-OCH2CH2-OSO2-Ph-CH3),7.35 (2H, d, -OSO2-Ph-CH3),7.80 (2H, d, -OSO2-Ph-CH3)MS (ESI+): Compound 42 542.4 [M+NH4]+; Crude productMS (ESI+): Compound 42 542.4 [M+NH4]+, Compound 1 212.7 [M+NH4]+, Compound 9 564.5 [M+NH4]+Yield: 52.0 g (yield: 89%)Purity: 96.7% (HPLC-RI)The HPLC measurement conditions used for the purity measurement are shown below.Apparatus: alliance manufactured by Waters Corporation.Column: Inertsil ODS-3 (column size: 4.6 mm×25 cm, particle size 5 μm) manufactured by GL Science Inc.Detector: RIDeveloping solvent: a solution of methanol/5 mM ammonium acetate=50/50, Flow rate: 0.6 mL/minColumn temperature: 40° C.Sample concentration: 0.2 mg/mLInjection volume: 40 μLThe purity value is the ratio of the peak area of the compound 42 to the total peak area detected over the retention time of 10 to 40 min.
References:
US2022/153683,2022,A1 Location in patent:Paragraph 0093-0122; 0337-0349; 0407-0445-0470
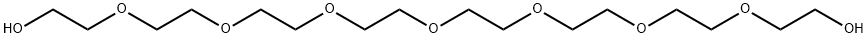
5117-19-1
228 suppliers
$14.00/100mg

98-59-9
585 suppliers
$9.00/5g

886469-23-4
38 suppliers
$198.00/100mg
![Bis[2-(2-hydroxyethoxy)ethyl] ether](/CAS/GIF/112-60-7.gif)
112-60-7
347 suppliers
$7.00/25g
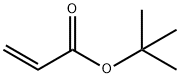
1663-39-4
234 suppliers
$10.00/5g

76-83-5
688 suppliers
$6.00/25g

98-59-9
585 suppliers
$9.00/5g

756526-06-4
97 suppliers
$76.00/100mg

886469-23-4
38 suppliers
$198.00/100mg
![Bis[2-(2-hydroxyethoxy)ethyl] ether](/CAS/GIF/112-60-7.gif)
112-60-7
347 suppliers
$7.00/25g

886469-23-4
38 suppliers
$198.00/100mg