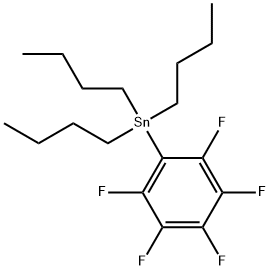
Pentafluorophenyltributylstannane synthesis
- Product Name:Pentafluorophenyltributylstannane
- CAS Number:1045-56-3
- Molecular formula:C18H27F5Sn
- Molecular Weight:457.11
Yield:1045-56-3 98%
Reaction Conditions:
bis(1,5-cyclooctadiene)nickel (0);tris(1-methylethyl)phosphine at 80; for 3 h;Product distribution / selectivity;
Steps:
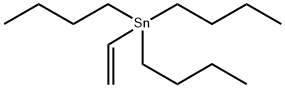
In a first example process, a single-step catalytic stannylation of a fluorinated arene compound was achieved using Bu3Sn(CHCH2) or Me3Sn(CHCH2) as a first organometallic compound in the presence of Ni(COD)2 with at least one of MeNC5H4NiPr and PiPr3 as a catalyst as follows: The preferred reaction advantageously shows quantitative functionalization and may be performed using as little as 1 mol % of Ni(COD)2 and MeNC5H4NiPr to go to completion. Test reactions were run at room temperature and yielded ethylene as a by-product. The reaction may further be performed without addition of solvent.In test samples the above reaction has been demonstrated to work with a number of different fluorinated arene compounds yielding resulting functionalized products in excess of 90%.Table 1 below illustrates example single-step reactions of the present invention involving use of fluorinated arene compounds having 2 to 5 fluorine substituents, the catalyst Ni(COD)2 and the ancillary ligand MeNC5H4NiPr and/or PiPr3. The yield percent marked with the superscript a provides NMR yield from integration of 19F[1H] NMR spectra, and yield percent marked with the superscript b provides isolated yield after chromatography. The condition hours marked with the superscript c and d denote that the reaction was carried out using 2.5 molar amount of Bu3Sn(CHCH2) and 10 fold excess of the fluorinated arene compound, respectively.
References:
US2011/282087,2011,A1 Location in patent:Page/Page column 4