
PENTYL HEXANOATE synthesis
- Product Name:PENTYL HEXANOATE
- CAS Number:540-07-8
- Molecular formula:C11H22O2
- Molecular Weight:186.29
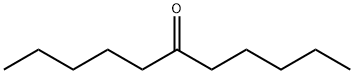
927-49-1
146 suppliers
$13.43/1gm:

540-07-8
117 suppliers
$15.00/5g
Yield:540-07-8 59%
Reaction Conditions:
with dihydrogen peroxide;lithium tetrakis(pentafluorophenyl)borate in water;1,2-dichloro-ethane at 50; for 55 h;Reagent/catalyst;
Steps:
73
As shown in Tables 5 and 6, the Baeyer-Villiger oxidation reaction was investigated using various reactive substrates. As the catalyst, a borate salt of Li or Ca was used. In general, although the catalytic activity of a Ca borate salt was higher, since a Li borate salt was a commercially available product, and the amount thereof to be used was small due to its low molecular weight, the Li borate salt was first used (Examples 57, 58, 66 to 71, and 73). However, when the Li borate salt was not sufficient depending on the reactive substrate, and the chemical yield of the product was low, the Ca borate salt was used as the catalyst (Examples 59 to 65, 72, and 74 to 76). In Example 73, when a chain ketone was used as the reactive substrate, a corresponding chain ester was obtained at a high yield. In Example 74, when a benzaldehyde, which was an aromatic aldehyde, was used as the reactive substrate, a corresponding formate was hydrolyzed in the system, and as a result, a corresponding phenol was obtained at a high yield. Product of Example 73: Colorless oil. TLC, Rf=0.73 (hexane-EtOAc=4:1); 1H NMR (CDCl3, 400 MHz) δ 0.88-0.95 (m, 6H), 1.28-1.39 (m, 8H), 1.58-1.66 (m, 4H), 2.29 (t, J=7.4 Hz, 2H), 4.06 (t, J=6.4 Hz, 2H); 13C NMR (CDCl3, 100 MHz) δ 14.0(2C), 22.5(2C), 24.8, 28.3, 28.6, 31.5, 34.5, 64.4, 173.9.
References:
NATIONAL UNIVERSITY CORPORATION NAGOYA UNIVERSITY;Ishihara, Kazuaki;Uyanik, Muhammet US2013/217898, 2013, A1 Location in patent:Paragraph 0070; 0076; 0098; 0100

927-49-1
146 suppliers
$13.43/1gm:

540-07-8
117 suppliers
$15.00/5g
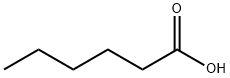
142-62-1
443 suppliers
$13.80/2.5g

71-41-0
425 suppliers
$9.33/100 mL

142-62-1
443 suppliers
$13.80/2.5g

540-07-8
117 suppliers
$15.00/5g
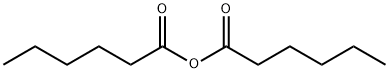
2051-49-2
174 suppliers
$16.00/25mL

540-07-8
117 suppliers
$15.00/5g

71-41-0
425 suppliers
$9.33/100 mL

142-62-1
443 suppliers
$13.80/2.5g

927-49-1
146 suppliers
$13.43/1gm:

540-07-8
117 suppliers
$15.00/5g