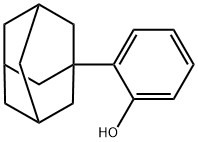
Phenol, 2-tricyclo[3.3.1.13,7]dec-1-yl- synthesis
- Product Name:Phenol, 2-tricyclo[3.3.1.13,7]dec-1-yl-
- CAS Number:38614-06-1
- Molecular formula:C16H20O
- Molecular Weight:228.33
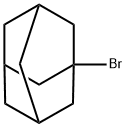
768-90-1
396 suppliers
$10.00/5g

108-95-2
725 suppliers
$14.00/25g
![Phenol, 2-tricyclo[3.3.1.13,7]dec-1-yl-](/CAS/20200611/GIF/38614-06-1.gif)
38614-06-1
9 suppliers
inquiry
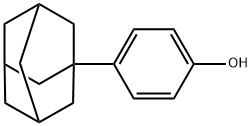
29799-07-3
133 suppliers
$9.00/250mg
Yield:29799-07-3 83% ,38614-06-1 17%
Reaction Conditions:
with molybdenum hexacarbonyl at 120; for 2 h;Sealed tube;regioselective reaction;
Steps:
Alkylation of aromatic compound with 1-bromoadamantane. General procedure.
General procedure: Into a pressure microreactor of stainless steel of capacity 17 mL or into a glass ampule of capacity 10 mL (the results of parallel runs differed insignificantly) was charged 1-3 mmol of catalyst [Mo(CO)6, MoO2(acac)2, Cr(CO)6,CoBr2, CuBr, CuBr2, W(CO)6], 100 mmol of 1-bromoadamantane, and 500 mmol of aromatic compound, the reactor was hermetically closed (the ampule was sealed) and heated at 110-130° for 1-3 h. On completing the reaction the reactor (ampule) was cooled to room temperature, opened, the reaction mixture was neutralized with NaHCO3 and filtered through a bed of Al2O3. The unreacted aromatic compound was distilled off, the residue was distilled in propanol).
References:
Khusnutdinov;Shchadneva;Khisamova [Russian Journal of Organic Chemistry,2015,vol. 51,# 11,p. 1545 - 1550][Zh. Org. Khim.,2015,vol. 51,# 11,p. 1576 - 1581,6]
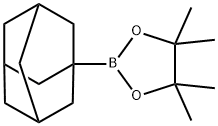
1357000-33-9
6 suppliers
inquiry
![Phenol, 2-tricyclo[3.3.1.13,7]dec-1-yl-](/CAS/20200611/GIF/38614-06-1.gif)
38614-06-1
9 suppliers
inquiry
768-95-6
418 suppliers
$10.00/5g

108-95-2
725 suppliers
$14.00/25g
![Phenol, 2-tricyclo[3.3.1.13,7]dec-1-yl-](/CAS/20200611/GIF/38614-06-1.gif)
38614-06-1
9 suppliers
inquiry

29799-07-3
133 suppliers
$9.00/250mg
935-56-8
187 suppliers
$37.00/5g

108-95-2
725 suppliers
$14.00/25g
![Phenol, 2-tricyclo[3.3.1.13,7]dec-1-yl-](/CAS/20200611/GIF/38614-06-1.gif)
38614-06-1
9 suppliers
inquiry

29799-07-3
133 suppliers
$9.00/250mg