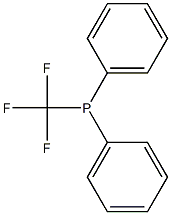
Phosphine, diphenyl(trifluoromethyl)- synthesis
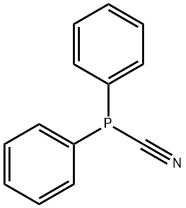
- Product Name:Phosphine, diphenyl(trifluoromethyl)-
- CAS Number:1605-56-7
- Molecular formula:C13H10F3P
- Molecular Weight:254.1875
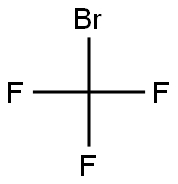
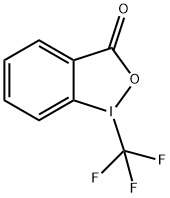
75-63-8
1 suppliers
inquiry
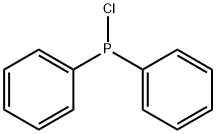
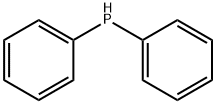
1079-66-9
425 suppliers
$5.00/5g
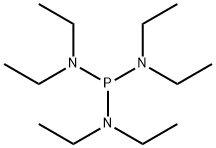
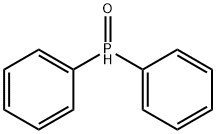
2283-11-6
169 suppliers
$30.00/1g

1605-56-7
1 suppliers
inquiry
Yield: 68%
Reaction Conditions:
in dichloromethane
Steps:
Diphenyltrifluoromethvlphosohine
EXAMPLES Diphenyltrifluoromethvlphosohine A flask equipped with a dry ice condenser was flame dried under a nitrogen stream, and charged with 3.1 mL (17 mmol) of chlorodiphenylphosphine and 10 mL of dichloromethane. After cooling the resulting solution to -78° C. and charging the condenser with dry ice and acetone, 4 mL (43 mmol) of bromotrifluoromethane (Freon 13B1) that had been condensed into a graduated tube was warmed to room temperature and allowed to distill into the flask. The cold solution was treated dropwise with 7 mL (26 mmol) of hexaethylphosphorous triamide, allowed to stir at "78° C. for 1 hour, and allowed to stir at room temperature overnight. Dilution with 50 mL of dichloromethane, water washing (three 50 mL portions), drying (MgSO4), and concentration afforded a brown liquid which was purified by flash chromatography on 50g of silica gel (230-400 mesh) eluted with petroleum ether to give 2.9g (68% yield) of diphenyltrifluoromethylphosphine as a colorless liquid. 19 F NMR (CDCl3, relative to CFCl3) -55.60 ppm (d, JPF =73 Hz); 31 P NMR (CDCl3, relative to H3 PO4) 3.11 ppm (quartet of pentets, JPF =73 Hz, JPH =8 Hz); mass spectrum (70 eV) m/z (relative intensity) 254 (41, M+), 185 (74), 183 (100), 127 (23), 107 (40), 77 (26), 69 (65), 51 (54).
References:
Ethyl Corporation US5008417, 1991, A
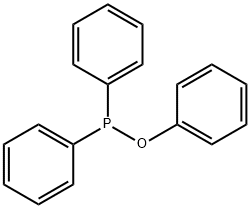
13360-92-4
98 suppliers
$6.00/250mg
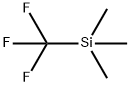
81290-20-2
402 suppliers
$13.00/1g

1605-56-7
1 suppliers
inquiry