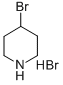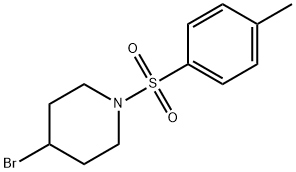
Piperidine, 4-bromo-1-[(4-methylphenyl)sulfonyl]- synthesis
- Product Name:Piperidine, 4-bromo-1-[(4-methylphenyl)sulfonyl]-
- CAS Number:347885-68-1
- Molecular formula:C12H16BrNO2S
- Molecular Weight:318.23
Yield:347885-68-1 97%
Reaction Conditions:
with pyridine at 20 - 30; for 24 h;Inert atmosphere;
Steps:
1 Preparation Example 1
Under the protection of argon, add 4-bromopiperidine hydrogen bromide (2.45g, 10mmol) to the reaction flask equipped with a stir bar, pyridine (30mL) is fully dissolved, and p-toluenesulfonyl chloride (2.86 g, 15mmol), react at room temperature for 24h. Add deionized water to the system to quench the reaction, spin off a large amount of pyridine, 1M hydrochloric acid/dichloromethane to extract the organic phase, dry with anhydrous sodium sulfate, concentrate, silica gel column chromatography, eluent is petroleum ether: ethyl acetate: Dichloromethane=10:1:1 to 10:1:2, the product is a white solid 3.10g, the yield is 97%, and the 1H NMR purity is greater than 98%.
References:
CN112142544,2020,A Location in patent:Paragraph 0106-0109
![4-Piperidinol, 1-[(4-methylphenyl)sulfonyl]-, 4-(4-methylbenzenesulfonate)](/CAS/20210305/GIF/101767-94-6.gif)
101767-94-6
0 suppliers
inquiry
![Piperidine, 4-bromo-1-[(4-methylphenyl)sulfonyl]-](/CAS/20210111/GIF/347885-68-1.gif)
347885-68-1
1 suppliers
inquiry
![1-[(4-METHYLPHENYL)SULFONYL]-4-PIPERIDINECARBOXYLIC ACID](/CAS/GIF/147636-36-0.gif)
147636-36-0
61 suppliers
$45.00/50mg
![Piperidine, 4-bromo-1-[(4-methylphenyl)sulfonyl]-](/CAS/20210111/GIF/347885-68-1.gif)
347885-68-1
1 suppliers
inquiry