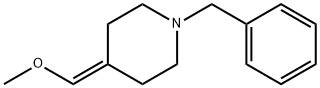
Piperidine, 4-(methoxymethylene)-1-(phenylmethyl)- synthesis
- Product Name:Piperidine, 4-(methoxymethylene)-1-(phenylmethyl)-
- CAS Number:120014-33-7
- Molecular formula:C14H19NO
- Molecular Weight:217.31
Yield:120014-33-7 33%
Reaction Conditions:
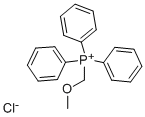
Stage #1: (methoxymethyl)triphenylphosphonium chloridewith n-butyllithium in diethyl ether;hexane at 20; for 0.5 h;
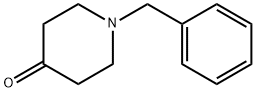
Stage #2: 1-phenylmethyl-4-piperidone in diethyl ether;hexane at 0 - 20; for 3 h;
Steps:
1.a
26.0 g of methoxymethylenetriphenylphosphonium chloride was suspended in 200 ml anhydrous ether, and 1.6 M n-butyllithiumhexane solution was added dropwise at room temperature. After stirring at room temperature for 30 minutes, the resultant was cooled to 0° C., and 14.35 g 1-benzyl-4-piperidone in 30 ml anhydrous ether solution was added. After stirring at room temperature for 3 hours, insoluble matter was filtered out and the filtrate was concentrated under reduced pressure. The obtained residue was dissolved in ether and extracted with IN hydrochloric acid. Following adjustment of pH to 12 with sodium hydroxide solution, the resultant was extracted with methylene chloride. The resultant was dried with magnesium sulfate, and concentrated under reduced pressure. The obtained residue was purified through a silica gel column, thereby obtaining 5.50 g of oily substance (yield 33%). Subsequently, the obtained oily substance was dissolved in 40 ml methanol, and added with 40 ml 1N hydrochloric acid. The reaction solution was heated to reflux for 3 hours, then concentrated under reduced pressure. The residue was dissolved in water. Thereafter, pH of the dissolved solution was adjusted to 12 with sodium hydroxide solution, and extracted with methylene chloride. The extracted solution was washed with saturated saline, dried with magnesium sulfate, and concentrated under reduced pressure. The obtained residue was purified through a silica gel column to obtain 2.77 g of 1-benzyl-4-piperidinecarboaldehyde (yield 54%) as an oily substance. The structure of the obtained compound was determined by NMR. Molecular formula; C13H17NO1H-NMR (CDCl3)δ; 1.40-2.40 (7H, m), 2.78 (2H, dt), 3.45 (2H, s), 7.20 (5H, s), 9.51 (1H, d).
References:
US2006/135507,2006,A1 Location in patent:Page/Page column 18

79-37-8
460 suppliers
$17.67/10gm:
67686-01-5
142 suppliers
$14.00/1g

120014-33-7
9 suppliers
inquiry