
Potassium cinnamate synthesis
- Product Name:Potassium cinnamate
- CAS Number:16089-48-8
- Molecular formula:C9H7KO2
- Molecular Weight:186.25
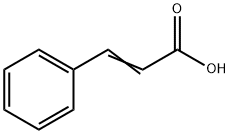
621-82-9
345 suppliers
$29.12/100G

16089-48-8
240 suppliers
$16.00/1g
Yield:-
Reaction Conditions:
with potassium hydroxide in water; pH=8.5 - 9.5Product distribution / selectivity;
Steps:
1
Experiment 1: Example of preparation of potassium cinnamate via a spray-drying process. A solution comprising about 14.5 wt% potassium cinnamate is made via reaction in an aqueous environment of an aqueous 50wt% potassium hydroxide solution with pure cinnamic acid powder at a final pH of about 8.5 to 9.5 for a 10 wt% solution. The solution was fed to a commercially available spray dryer. The solution was fed with an ingoing temperature of about 55° C and atomized by means of using pressure nozzles (∼30 bar). The spray of droplets was brought in contact with heated air with an inlet temperature of about 180°C. The outlet temperature was about 95°C. The resulting potassium cinnamate powder had an average moisture content of about less than 2wt%.
References:
PURAC Biochem BV EP2330094, 2011, A1 Location in patent:Page/Page column 4