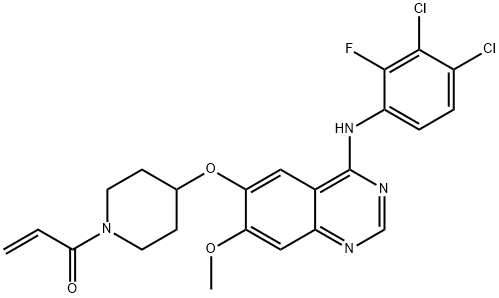
Poziotinib (HM781-36B) synthesis
- Product Name:Poziotinib (HM781-36B)
- CAS Number:1092364-38-9
- Molecular formula:C23H21Cl2FN4O3
- Molecular Weight:491.34

1429757-65-2
42 suppliers
inquiry
![1-[4-[[(4-Methylphenyl)sulfonyl]oxy]-1-piperidinyl]-2-propen-1-one](/CAS/20200611/GIF/1620740-65-9.gif)
1620740-65-9
4 suppliers
inquiry

1092364-38-9
184 suppliers
$30.00/1mg
Yield:1092364-38-9 77%
Reaction Conditions:
with potassium carbonate in N,N-dimethyl acetamide at 70; for 24 h;
Steps:
1.1-2 Preparation of l-(4-(4-(3,4-dichloro-2-fluorophenylamino)-7- methoxyquinazolin-6-yloxy)piperidin-l-yl)prop-2-en-l-one, the compound of formula (I)
4-(3,4-dichloro-2-fluorophenylamino)-7-methoxyquinazolin-6-ol (12 g, 34 mmol) prepared in Preparation Example 1, l-acryloylpiperidin-4-yl 4- methylbenzenesulfonate (16 g, 51 mmol) prepared in step (1-1), K2CO3 (9.4 g, 68 mmol) and dimethylacetamide (DMAc, 300 mL) were admixed. The reaction temperature was raised to 70°C, and the mixture was stirred for 24 hours. Upon completion of the reaction, the mixture was cooled down to room temperature, extracted with ethyl ester (300 mL), and then washed with water (300 mL). The organic layer was separated, and distilled under a reduced pressure. The residue thus obtained was solidified by adding ethyl ester, filtered, and dried to obtain the target compound (12.8 g, yield: 77%). 1H-NMR (300 MHz, DMSO-d6) δ 9.65 (bs, 1H), 8.40 (s, 1H), 7.88 (s, 1H), 7.64-7.56 (m, 2H), 7.24 (s, 1H), 6.89-6.80 (m, 1H), 6.15-6.08 (m, 1H), 5.70-5.66 (m, 1H), 4.78 (m, 1H), 3.94 (s, 3H), 3.87 (m, 2H), 3.48 (m, 2H), 2.03 (m, 2H), 1.70 (m, 1H).
References:
WO2014/116070,2014,A1 Location in patent:Page/Page column 10-11

1429757-65-2
42 suppliers
inquiry
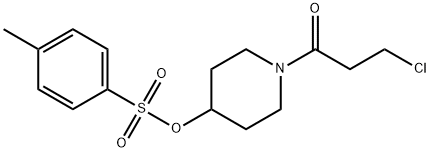
1620740-66-0
1 suppliers
inquiry

1092364-38-9
184 suppliers
$30.00/1mg
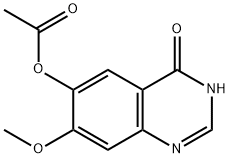
179688-53-0
278 suppliers
$6.00/1g

1092364-38-9
184 suppliers
$30.00/1mg
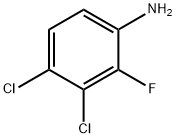
886762-39-6
99 suppliers
$6.00/1g

1092364-38-9
184 suppliers
$30.00/1mg
![6-Quinazolinol, 4-[(3,4-dichloro-2-fluorophenyl)amino]-7-methoxy-, 6-acetate](/CAS/20200515/GIF/1429757-64-1.gif)
1429757-64-1
14 suppliers
inquiry

1092364-38-9
184 suppliers
$30.00/1mg