
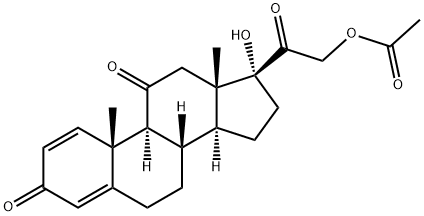
Prednisone 17, 21-Diacetate synthesis
- Product Name:Prednisone 17, 21-Diacetate
- CAS Number:6677-19-6
- Molecular formula:C25H30O7
- Molecular Weight:442.5
125-10-0
339 suppliers
$39.00/10mg

64-19-7
1553 suppliers
$10.00/25ML

6677-19-6
15 suppliers
$625.00/500mg
Yield:6677-19-6 100%
Reaction Conditions:
with trifluoroacetic anhydride;toluene-4-sulfonic acid at 0 - 20; for 3.5 h;
Steps:
1
Example 1Preparation of acetic acid 2-((10R,13S,17R)-17-acetoxy-10,13- dimethyl-3,ll-dioxo 6,7,8,9,10,H,12,13,14,15,16,17-dodecahydro-3H- cyclopenta[a]phenanthren-17-yl)-2-oxo-ethyl ester (intermediate 2)To a suspension of acetic acid 2-((10R, 13S, 17R)-17-hydroxy- 10, 13- dimethyl-3, l l-dioxo-6,7,8,9, 10, l 1 , 12, 13, 14, 15, 16, 17-dodecahydro-3H- cyclopenta[a]phenanthren-17-yl)-2-oxo-ethyl ester (intermediate 1) (2 g, 4.99 mmol) and p-toluene sulphonic acid (PTSA) (200 mg, 1.051 mmol) in acetic acid (5 ml), at 0°C, trifluoroacetic anhydride (5 ml, 35.4 mmol) was slowly added over 10 minutes period. After stirring at 0°C for 20 min, the reaction mixture was stirred at RT for 3 hr.The reaction mixture was poured in ice/water (130 ml) and the resulting mixture was extracted with DCM (2 x 100 ml) and AcOEt (2 x 100 ml). The combined organic extracts were dried over anhydrous Na2SO4 and concentrated. The crude product was purified by flash chromatography on silica gel, in gradient elution from DCM to DCM AcOEt 50: 50 to give the title compound (2.64 g, quantitative yield).LC-MS (ESI POS): 445.2 (MH+)
References:
WO2011/29547,2011,A2 Location in patent:Page/Page column 43

53-03-2
400 suppliers
$28.00/1g

108-24-7
5 suppliers
$14.00/250ML

6677-19-6
15 suppliers
$625.00/500mg