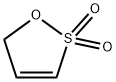
Prop-1-ene-1,3-sultone synthesis
- Product Name:Prop-1-ene-1,3-sultone
- CAS Number:21806-61-1
- Molecular formula:C3H4O3S
- Molecular Weight:120.13

10200-48-3
4 suppliers
inquiry

21806-61-1
186 suppliers
$6.00/250mg
Yield:21806-61-1 94%
Reaction Conditions:
with methanesulfonyl chloride;triethylamine in ethyl acetate at -20 - 10; for 4 h;Cooling with ice;
Steps:
4
Example 4Synthesis of 1,3-propenesultone (the third step, Reaction Scheme [V]) 2-Hydroxy-1,3-propanesultone (1.70 g, 12.3 mmol) obtained according toExample 3 was dissolved in ethyl acetate (12 mL), and thereafter triethylamine (Et3N) (2.99 g, 29.5 mmol, produced by Wako Pure Chemical Industries, Ltd.) and methanesulfonyl chloride (MsCl) (1.69 g, 14.7 mmol, produced by Wako Pure Chemical Industries, Ltd.) were added thereto under ice-cooling. The solution was stirred at -20° C. to 10° C. for 4 hours to promote the reaction. After completion of the reaction, water (12 mL) was added to the reaction solution, and then the solution was stirred. Subsequently, the organic layer was separated. After the separated organic layer was washed with water, the organic layer was concentrated, thereafter toluene was added to the concentrated residue. The crystal formed was filtered, and the resultant crystal was dried, to obtain 1,3-propenesultone (1.39 g, yield: 94%) relevant to the above-described general formula [4] as a white crystal. Measurement results of 1H-NMR are shown below.1H-NMR (400 MHz, CDCl3) 6 (ppm): 5.12 (1H), 6.81 (1H), 7.00 (1H).
References:
US2012/130089,2012,A1 Location in patent:Page/Page column 10

63145-09-5
2 suppliers
inquiry

21806-61-1
186 suppliers
$6.00/250mg
1263199-75-2
1 suppliers
inquiry

21806-61-1
186 suppliers
$6.00/250mg
![4-BROMO-[1,2]OXATHIOLANE 2,2-DIOXIDE](/CAS/GIF/189756-89-6.gif)
189756-89-6
26 suppliers
inquiry

21806-61-1
186 suppliers
$6.00/250mg

7459-72-5
31 suppliers
inquiry

21806-61-1
186 suppliers
$6.00/250mg