
prop-1-ene-2-sulfonyl chloride synthesis
- Product Name:prop-1-ene-2-sulfonyl chloride
- CAS Number:874009-75-3
- Molecular formula:C3H5ClO2S
- Molecular Weight:140.59
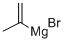
13291-18-4
174 suppliers
$133.00/25ml

874009-75-3
8 suppliers
inquiry
Yield:-
Reaction Conditions:
Stage #1: isopropenylmagnesium bromidewith sulfuryl dichloride in hexanes;diethyl ether at 0 - 20;
Stage #2: with water in hexanes;diethyl ether;
Steps:
prop-1-ene-2-sulfonamide, A 0.5 M solution of prop-1-en-2-ylmagnesium bromide (20 mL 10.0 mmol) in Et2O was added slowly to a stirring solution of sulfuryl dichloride (1.6 mL, 20 mmol) in hexanes (20 mL) at 0° C. The reaction was slowly allowed to warm to rt and stirred 16 h. The reaction was quenched with water (40 mL), the layers separated and the organic layer was concentrated to a clear liquid. The residue was dissolved into THF (30 mL), cooled to 0° C. and treated with ammonia (10 mL) using a cold finger (-70° C.). The cooling bath was removed and the reaction was allowed to stir at rt for 1 h with the cold finger attached, and then stirred open to air at rt ON. The reaction was filtered, concentrated under vacuum and partially purified through a pad of silica (eluting with EtOAc/hexanes 1:1). Fractions containing product were combined, concentrated and then recrystallized from hexanes/EtOAc (3:1) to yield prop-1-ene-2-sulfonamide (161 mg, 1.33 mmol, 13%) as a white solid. 1H NMR (300 MHz, CDCl3) δ ppm 6.06 (s, 1H), 5.55 (q, J=1.5 Hz, 1H), 4.57 (br s, 2H), 2.14 (, br s, 3H).
References:
US2010/216774,2010,A1 Location in patent:Page/Page column 62
16621-26-4
0 suppliers
inquiry

874009-75-3
8 suppliers
inquiry