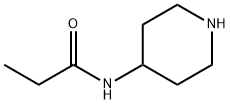
Propanamide, N-4-piperidinyl- synthesis
- Product Name:Propanamide, N-4-piperidinyl-
- CAS Number:139112-22-4
- Molecular formula:C8H16N2O
- Molecular Weight:156.23
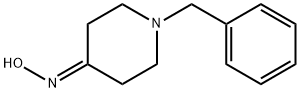
![Propanamide, N-[1-(phenylmethyl)-4-piperidinyl]-](/CAS/20210305/GIF/139062-93-4.gif)
139062-93-4
0 suppliers
inquiry

139112-22-4
12 suppliers
inquiry
Yield:139112-22-4 99%
Reaction Conditions:
with palladium 10% on activated carbon;hydrogen;palladium(II) hydroxide in ethanol; under 2585.81 Torr; for 24.75 h;Inert atmosphere;
Steps:
Synthesis of N-(piperidin-4-yl)propionamide
0.7 g of N-(1-benzylpiperidin-4-yl)propionamide (1 equivalent, 0.003 moles) were added to a parr hydrogenation flask and dissolved in 30 mL of EtOH. The solution was then degassed with argon for 30 min followed by the addition of 0.07 g of 10% Pd/C (0.2 equiv., 6.58 x 10"4 mol) and 0.07 g of 20% Pd(OH)2 (0.17 equiv., 4.98 x 10-4 mol). The black solution was then degassed with argon for an additional 1 5 min. The reaction mixture was then charged with 50 psi of H2 gas and shaken for 24 h. The product was filtered through celite and the solvent was removed via rotary evaporation. No further purification was required. Yield: 0.467 g (99%)
References:
WO2016/29218,2016,A1 Location in patent:Page/Page column 14
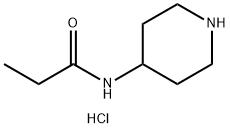
183732-59-4
11 suppliers
$237.00/500mg

139112-22-4
12 suppliers
inquiry
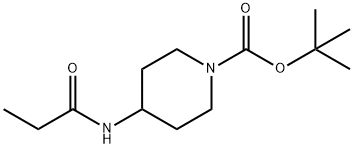
1233954-94-3
6 suppliers
$330.00/1g

139112-22-4
12 suppliers
inquiry
949-69-9
108 suppliers
$28.60/10MG

139112-22-4
12 suppliers
inquiry
3612-20-2
488 suppliers
$6.00/10g

139112-22-4
12 suppliers
inquiry