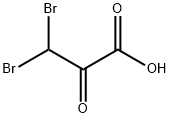
Propanoic acid, 3,3-dibromo-2-oxo-, methyl ester synthesis
- Product Name:Propanoic acid, 3,3-dibromo-2-oxo-, methyl ester
- CAS Number:5038-58-4
- Molecular formula:C4H4Br2O3
- Molecular Weight:259.88
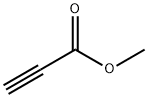
922-67-8
343 suppliers
$6.00/1g

5038-58-4
1 suppliers
inquiry
Yield:5038-58-4 49%
Reaction Conditions:
with N-Bromosuccinimide;iron(III) chloride hexahydrate;water in tetrahydrofuran at 80; for 3 h;Inert atmosphere;
Steps:
General procedure for products 4 (Table 3)
General procedure: To a mixture of alkyne 1 (0.5 mmol), N-halosuccinimide 2 (2.0 mmol), and FeCl3·6H2O (0.025 mmol) was added water (1.0 mL) and tetrahydrofuran (THF, 2.0 mL) under nitrogen at room temperature. The reaction temperature was raised to 80 °C for 3 h. The temperature of the reaction was cooled to room temperature. The resulting reaction solution was quenched with 2 mL of saturated NaHCO3 and extracted with 15 mL of ethyl acetate for three times. The extract was dried over MgSO4. After short column chromatography, the solvent was evaporated in vacuo to afford the crude products. NMR yields were determined by 1H NMR using dibromomethane as an internal standard. Solvent was evaporated, and the residue was purified by flash column chromatography on silica gel with ethyl acetate/petroleum ether (1:200) as an eluent.
References:
Liu, Jinhua;Li, Wenjuan;Wang, Chao;Li, Yao;Li, Zhiping [Tetrahedron Letters,2011,vol. 52,# 33,p. 4320 - 4323] Location in patent:supporting information; experimental part