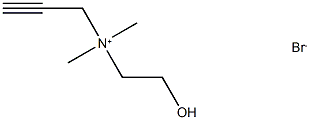
Propargylcholine synthesis
- Product Name:Propargylcholine
- CAS Number:111755-76-1
- Molecular formula:C7H14BrNO
- Molecular Weight:208.0962
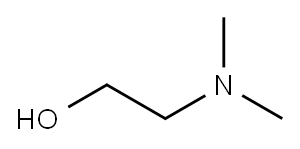
108-01-0
19 suppliers
$10.00/20mg

106-96-7
331 suppliers
$30.00/25g

111755-76-1
30 suppliers
$45.00/10mg
Yield:-
Reaction Conditions:
in tetrahydrofuran;toluene at 20;Inert atmosphere;
Steps:
Propargyleboline synthesis.
Propargvlchoiine was synthesized according to Jao, C.Y., Roth, M., Wehi, R. & Salic, A.. Proc. MiL Acrid. SeE. LISA 1(16, 1533245337 (2009). 3 mL propargyl bromide (80 vt, % solution in toluene) were added dropwise to 3 g 2-dimethylaminoethanol in 10 mL anhydrous THE on ice under argon gas protection and stirring. The ice bath was removed and the mixture was kept stirring at room temperature overnight. The white solids were filtered the next day and washed extensively with cold anhydrous YEW to obtain 5 g pure propargylcholine bromide All chemicals here are Purchased from Sigma-Aldrich. NMR. spectrum was recorded on a Bruker 400 (400?1Hz) Fourier Transform (FT) NMR spectrometers at the Columbia University Chemistry Department. 1FI NM.R spectra are tabulated in the following order: multiplicity (s, singlet; ci, doublet; t, triplet; m, multipietl, number of protons. ti NMR (400 MHz, D0) 6 ppm: 4.37 (d. J 2.4 Hz, 214); 4.10 (in, 211); 3.66 (t, .J 4.8 Hz, 2Ff); 3.28 (s, 611); MS (APCI+) mlz Caled. for C7H4N0 [Mi:12. 19. Found: 128.26.
References:
THE TRUSTEES OF COLUMBIA UNIVERSITY IN THE CITY OF NEW YORK;MIN, Wei;WEI, Lu;CHEN, Zhixing;HU, Fanghao;SHEN, Yihui WO2014/205074, 2014, A2 Location in patent:Paragraph 0171
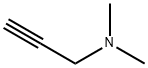
7223-38-3
89 suppliers
$23.00/5mL

540-51-2
258 suppliers
$17.00/5g

111755-76-1
30 suppliers
$45.00/10mg