
PROPYNAL synthesis
- Product Name:PROPYNAL
- CAS Number:624-67-9
- Molecular formula:C3H2O
- Molecular Weight:54.05

107-19-7
5 suppliers
$12.00/25g

624-67-9
52 suppliers
$120.00/500mg
Yield:624-67-9 90%
Reaction Conditions:
with chromium(VI) oxide;sulfuric acid in water;butanone at 20 - 25; under 760.051 Torr; for 1 h;
Steps:
1.A.6.1 A.6.1) Preparation of N-(4-methoxyphenyl)-prop-2-yn-1-imine
N-(4-methoxyphenyl)-prop-2-yn-1-imine is obtained in two steps. First, 2-propynal is obtained from 2-propyn-l-ol as described in Veliev et al., 1980, Synthesis 6, 461. 2-propyn-l -ol is dissolved in 2-butanone (C2H5COCH3) and oxidized by an aqueous solution of chromium trioxide (Cr03) and sulfuric acid (H2S04) at ambient temperature and atmospheric pressure. A solution of chromium trioxide (60 g) in sulfuric acid (40 ml) and water (120 ml) is added dropwise with stirring over 1 hour to a solution of 2-propyn-l-ol (35.88 g, 37.26 ml, 640 mmol) in 2-butanone (100 ml). The temperature is maintained at 20-25°C by cooling. The reaction mixture is stirred for 4 hours and diluted with water (30 ml). The organic layer is separated. The aqueous layer is extracted with diethyl ether (Et20, 120 ml). The combined organic layers are dried over anhydrous magnesium sulfate (MgS04). The solvents are removed in vacuo. Distillation of the residual liquid gives 2-propynal (31.13 g, 34.98 ml, 576 mmol, 90%).
References:
VITA API;ETAT FRANÇAIS REPRÉSENTÉ PAR LE DIRECTEUR CENTRAL DU SERVICE DE SANTÉ DES ARMÉES;UNIVERSITÉ D'AIX-MARSEILLE;CENTRE NATIONAL DE LA RECHERCHE SCIENTIFIQUE - CNRS -;SIMON, Philippe Yves-Rémy;OREAL, Henri;AUDRAN, Gérard;SCHULZ, Marvin;JOLY, Jean-Patrick;SIRI, Didier;SIRI, Anouk WO2018/115497, 2018, A1 Location in patent:Page/Page column 53; 54; 55

107-19-7
5 suppliers
$12.00/25g

624-67-9
52 suppliers
$120.00/500mg

471-25-0
232 suppliers
$10.00/1g

6921-27-3
78 suppliers
$23.00/1g

624-67-9
52 suppliers
$120.00/500mg

463-49-0
47 suppliers
$125.00/10 g
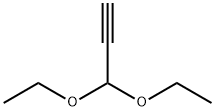
10160-87-9
208 suppliers
$8.00/1g

624-67-9
52 suppliers
$120.00/500mg
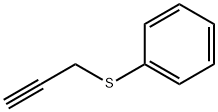
5651-88-7
54 suppliers
$58.00/5ml

624-67-9
52 suppliers
$120.00/500mg