
Allene synthesis
- Product Name:Allene
- CAS Number:463-49-0
- Molecular formula:C3H4
- Molecular Weight:40.06
Yield:-
Reaction Conditions:
with potassium hydroxide-supported silica under 60.006 Torr; for 1 h;Inert atmosphere;
Steps:
1 Example 1
Example 1[0104] When methyl methacrylate is produced using acetone, methanol, and carbon monoxide as starting materials,the production process can be carried out in the most suitable manner, for example, by flow in Fig. 1 and material balancein Table 1.[0105] Acetone (fluid number 1) (647 kg/h) was supplied to a dehydration reaction step (A), together with 926 kg/h ofa recycle fraction (fluid number 9) from an acetone circulation step (D) and then acetone is subjected to a dehydrationreaction in the presence of a potassium hydroxide-supported silica catalyst to obtain 1, 573 kg/h of a dehydration reaction mixture (fluid number 2) which contains 27.0% by weight in total of propyne and propadiene, and also contains theunreacted acetone, product water, by-products, and impurities in starting materials. The dehydration reaction mixture thus obtained is supplied to a propyne/propadiene separation step (B) and is partially condensed while cooling to therebyseparate into 461 kg/h of a gas mixture (fluid number 3) containing propyne and propadiene as main components, and1,112 kg/h of a liquid mixture (fluid number 4) containing water and acetone as main components. The gas mixture (fluidnumber 3) obtained in the propyne/propadiene separation step is supplied to a propyne purification step (C), and thenseparated into 23 kg/h of a gas mixture (fluid number 5) containing a low boiling component as a main component, 151kg/h of a fraction (fluid number 6) containing propadiene as a main component, 406 kg/h of purified propyne (fluid number7), and 32 kg/h of a fraction (fluid number 8) containing a high boiling component as a main component by cooling anddistillation. The liquid mixture (fluid number 4) obtained in the propyne/propadiene separation step (B) is supplied anacetone circulation step (D) and then separated into 926 kg/h of a recycle fraction (fluid number 9) containing acetoneas main component and 186 kg/h of water (fluid number 10) by distillation. The fraction (fluid number 6) containingpropadiene as a main component obtained in the propyne purification step (C) is supplied to an isomerization reactionstep (E) and then propadiene is subjected to an isomerization reaction in the presence of a potassium carbonate supported-alumina catalyst to obtain 151 kg/h of an isomerization reaction mixture (fluid number 11) containing propyne asa main component
References:
EP2960228,2015,A1 Location in patent:Paragraph 0104; 0105; 0109; 0110; 0111

67-64-1
6 suppliers
$19.10/10ml
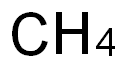
34557-54-5
0 suppliers
inquiry

463-49-0
46 suppliers
$125.00/10g

74-99-7
96 suppliers
$45.00/1ml

67-64-1
6 suppliers
$19.10/10ml
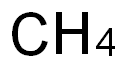
34557-54-5
0 suppliers
inquiry

463-49-0
46 suppliers
$125.00/10g
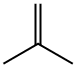
115-11-7
228 suppliers
$45.00/25g

74-99-7
96 suppliers
$45.00/1ml
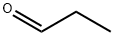
123-38-6
422 suppliers
$14.00/5mL

74-84-0
55 suppliers
$269.00/110g

463-49-0
46 suppliers
$125.00/10g
201230-82-2
1 suppliers
inquiry

74-99-7
96 suppliers
$45.00/1ml