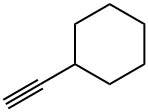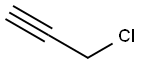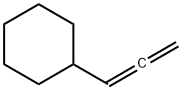
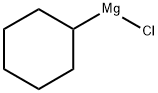
CYCLOHEXALALLENE synthesis
- Product Name:CYCLOHEXALALLENE
- CAS Number:5664-17-5
- Molecular formula:C9H14
- Molecular Weight:122.21
Yield:-
Reaction Conditions:
Stage #1:1-bromocyclohexane with magnesium;ethylene dibromide in tetrahydrofuran for 3 h;Reflux;
Stage #2: with copper(I) bromide;lithium bromide in tetrahydrofuran at -78; for 0.333333 h;
Stage #3:propargyl bromide in tetrahydrofuran;toluene at 20;
Steps:
9 Example 9
Under the protection of nitrogen, Add 18mmol of magnesium chips (435mg) to the three-neck bottle. Soluble in 20mL tetrahydrofuran, add 5mL to dissolve 0.75mmol 1,2-dibromoethane with 15 mmol of bromocyclohexane (2.45 g) in tetrahydrofuran, Stirring at reflux for 3 h, Produce format reagents, Cool it to -78 °C, 3.6 mmol LiBr (300 mg) and 1.05 mmol CuBr (150 mg) were added. Stirring at -78 °C for 20 min, Add 18 mmol of bromopropyne (2 mL, 80% in toluene), The reaction solution was warmed to room temperature. Stir overnight, After the reaction, it is quenched with a saturated ammonium chloride solution. Extracted with ether, To the obtained reaction liquid, 100-200 mesh column chromatography silica gel was added, and the solvent was distilled off under reduced pressure. The obtained crude product was separated by silica gel column chromatography. Elution was carried out using petroleum ether as an eluent to obtain an olefin represented by the formula 5c.
References:
Zhejiang University of Technology;Liu Yunkui;Bao Hanyang;Xu Zheng CN109422769, 2019, A Location in patent:Paragraph 0055-0057
931-51-1
127 suppliers
$42.00/100ml

106-96-7
332 suppliers
$30.00/25g

5664-17-5
16 suppliers
$81.90/1g

106-96-7
332 suppliers
$30.00/25g
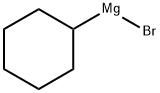
931-50-0
107 suppliers
$45.00/1g
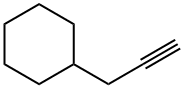
17715-00-3
81 suppliers
$39.89/1g

5664-17-5
16 suppliers
$81.90/1g