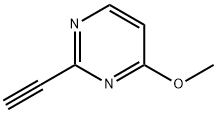
Pyrimidine, 2-ethynyl-4-methoxy- (9CI) synthesis
- Product Name:Pyrimidine, 2-ethynyl-4-methoxy- (9CI)
- CAS Number:161489-04-9
- Molecular formula:C7H6N2O
- Molecular Weight:134.14
![Pyrimidine, 4-methoxy-2-[2-(trimethylsilyl)ethynyl]-](/CAS/20210305/GIF/161489-00-5.gif)
161489-00-5
0 suppliers
inquiry

161489-04-9
14 suppliers
inquiry
Yield: 85%
Reaction Conditions:
Stage #1:4-Methoxy-2-<(trimethylsilyl)ethynyl>pyrimidine with tetrabutyl ammonium fluoride;acetic acid in tetrahydrofuran at 20; for 0.0833333 h;
Stage #2: with potassium carbonate in tetrahydrofuran;water
Steps:
1.b
Tetrabutylammonium fluoride (0.97 mL of a 1M solution in tetrahydrofuran, 0.97 mmol) was added to a stirred solution of 4-methoxy-2-[(trimethylsilyl)ethynyl]pyrimidine (Preparation 1a, 0.20 g, 0.97 mmol) in tetrahydrofuran (1.4 mL) and acetic acid (56 μ) at room temperature. After 5 minutes, 10% aqueous potassium carbonate solution was added to the reaction mixture and the mixture was extracted with dichloromethane. The organic layer was separated, dried (MgS04) and evaporated. Purification of the residue by flash chromatography (dichloromethane) gave the title compound (0.11 g, 85%) as a pale orange solid.LRMS (m/z): 135 (M+1)+.1H-NMR δ (300 MHz, CDCI3): 3.1 (s, 1H), 4.0 (s, 3H), 6.7 (d, 1 H), 8.4 (d, 1 H).
References:
ALMIRALL, S.A.;BACH TAŇA, Jordi;PAGES SANTACANA, Lluis, Miquel;TALTAVULL MOLL, Joan;EASTWOOD, Paul, Robert;GONZALEZ RODRIGUES, Jacob;GIULIO MATASSA, Victor WO2011/101161, 2011, A1 Location in patent:Page/Page column 82
22536-63-6
181 suppliers
$14.00/1g

161489-04-9
14 suppliers
inquiry