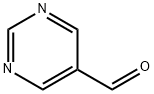
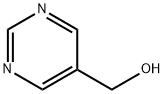
Pyrimidine-5-carboxaldehyde synthesis
- Product Name:Pyrimidine-5-carboxaldehyde
- CAS Number:10070-92-5
- Molecular formula:C5H4N2O
- Molecular Weight:108.1
626-55-1
536 suppliers
$5.00/5 / G
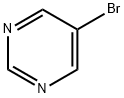
4595-59-9
459 suppliers
$6.00/1g
497-19-8
1378 suppliers
$13.00/300G
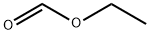
109-94-4
462 suppliers
$10.00/10g

10070-92-5
269 suppliers
$10.00/250mg
Yield:10070-92-5 35%
Reaction Conditions:
with hydrogenchloride;n-butyllithium in tetrahydrofuran;hexane;water
Steps:
15.1 1)
1) Preparation of pyrimidine-5-carbaldehyde A solution of 5-bromo-pyrimidine (5 g, 31.4 mmol, 1.0 eq.) in anhydrous THF (300 mL) was placed under an argon atmosphere in a three-necked round bottom flask equipped with a low-temperature thermometer. The mixture was cooled to -100°C in a EtOH/N2(1) bath. To the solution of 5-bromopyridine was added a solution of n-BuLi in hexane (20 mL, 1.6 M, 32.5 mmol, 1.0 eq.). The resulting mixture was stirred for 20 minutes at 100°C and the organolithium that formed was trapped with a solution of ethyl formate (2.7 mL, 33.5 mmol, 1.1 eq.) in THF (10 mL). The reaction was stirred for another 20 minutes at 100°C and quenched with a solution of hydrochloric acid in ether (17 mL, 2M, 34 mmol). Then, the cold bath was removed and the mixture stirred at room temperature for 1 hour. The solution was concentrated under reduced pressure, and then treated with water and saturated aqueous sodium carbonate (10 mL). The mixture was extracted with dichloromethane and the organic layer was dried over MgSO4. The solvent was removed under reduced pressure and the residue purified by silica gel flash-column chromatography (eluent: CH2Cl2/AcOEt, 60:40 to 50:50) to afford pyrimidine-5-carbaldehyde as beige crystals (1.2 g, 35%).
References:
Centre National de la Recherche Scientifique EP2266562, 2010, A1

4595-59-9
459 suppliers
$6.00/1g
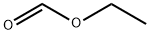
109-94-4
462 suppliers
$10.00/10g

10070-92-5
269 suppliers
$10.00/250mg
25193-95-7
148 suppliers
$16.00/250mg

10070-92-5
269 suppliers
$10.00/250mg
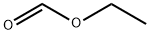
109-94-4
462 suppliers
$10.00/10g

10070-92-5
269 suppliers
$10.00/250mg

4595-59-9
459 suppliers
$6.00/1g
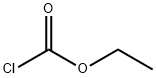
541-41-3
6 suppliers
$19.39/5g

10070-92-5
269 suppliers
$10.00/250mg