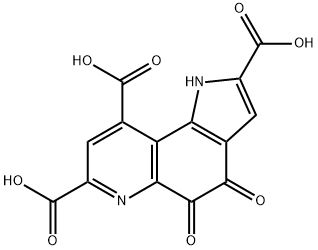
Pyrroloquinoline quinone synthesis
- Product Name:Pyrroloquinoline quinone
- CAS Number:72909-34-3
- Molecular formula:C14H6N2O8
- Molecular Weight:330.21
With sodium carbonate In water at 30℃; for 24h;
Or with water; potassium carbonate In water at 25 - 80℃; Green chemistry; Industrial scale.
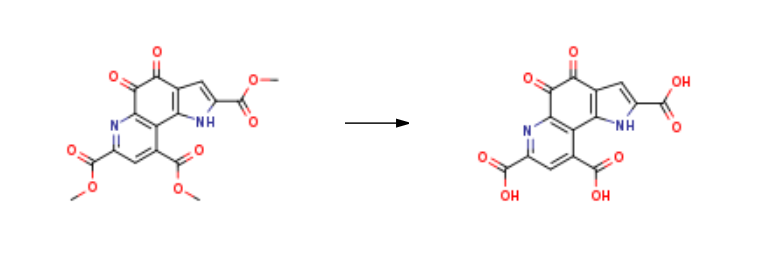
![7,9-DIMETHOXYCARBONYL-2-ETHOXYCARBONYL-1H-PYRROLO-[2,3-F]QUINOLINE-4,5-DIONE](/StructureFile/ChemBookStructure22/GIF/CB8648886.gif)
80721-47-7
12 suppliers
$432.00/5mg

72909-34-3
398 suppliers
$38.00/25mg
Yield:72909-34-3 97%
Reaction Conditions:
Stage #1: 4,5-Dihydro-4,5-dioxo-1H-pyrrolo<2,3-f>chinolin-2,7,9-tricarbonsaeure-(2-ethyl)(7,9-dimethyl)esterwith lithium hydroxide;water in tetrahydrofuran at 0 - 17; for 31.5 h;
Stage #2: with hydrogenchloride;potassium chloride in tetrahydrofuran;water at 0; pH=5.3 - 6; for 1 h;
Stage #3: with sulfuric acid in water at 20; for 2.5 h;
Steps:
1.J; j; k
Into a 1-L, 3-neck flask equipped with a mechanical stirrer, a temperature probe, an addition funnel, a nitrogen purge system, and an ice bath were placed, 4,5-dioxo-4,5-dihydro- EPO
References:
WO2006/102642,2006,A1 Location in patent:Page/Page column 17-18; 1/6; 2/6; 3/6
![4,5-Dioxo-4,5-dihydro-1H-pyrrol[2,3-f]quinoline-2,7,9-tricarboxylic acid trimethyl ester](/CAS/GIF/74447-88-4.gif)
74447-88-4
5 suppliers
inquiry

72909-34-3
398 suppliers
$38.00/25mg
![1H-Pyrrolo[2,3-f]quinoline-2,7,9-tricarboxylic acid, 4,5-dihydro-5,5-dimethoxy-4-oxo-, 2,7,9-trimethyl ester](/CAS/20210305/GIF/78939-40-9.gif)
78939-40-9
0 suppliers
inquiry

72909-34-3
398 suppliers
$38.00/25mg
![1H-Pyrrolo[2,3-f]quinoline-2,7,9-tricarboxylic acid, 5-methoxy-](/CAS/20210305/GIF/116451-31-1.gif)
116451-31-1
0 suppliers
inquiry

72909-34-3
398 suppliers
$38.00/25mg
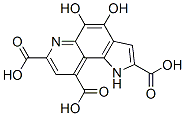
79127-57-4
2 suppliers
inquiry

72909-34-3
398 suppliers
$38.00/25mg