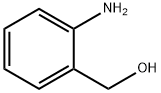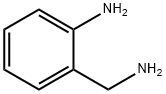
Quinoline, 2-(3-bromophenyl)- synthesis
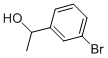
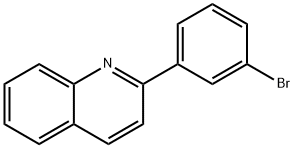
- Product Name:Quinoline, 2-(3-bromophenyl)-
- CAS Number:108062-54-0
- Molecular formula:C15H10BrN
- Molecular Weight:284.15
Yield:108062-54-0 85%
Reaction Conditions:
with [(p-cymene)Ru(BiBzImH2)Cl][Cl];caesium carbonate in tert-Amyl alcohol at 125; for 12 h;
Steps:
4.4. Procedure for acceptorless dehydrogenative coupling of ketoneswith o-aminobenzyl alcohols to quinolines catalyzed by [(p-cymene)Ru(BiBzImH2)Cl][Cl]
General procedure: In a round-bottomed flask with a condenser tube were added oaminobenzylalcohols 1 (1 mmol) and ketones 2 (1.2 mmol, 1.2equiv), cat. 1 (6 mg, 0.01 mmol, 1 mol %), Cs2CO3 (163 mg,0.5 mmol, 0.5 equiv) and tert-amyl alcohol (1 mL). The reactionmixture was heated at 125 C in an oil bath for 12 h and thencooled to ambient temperature, concentrated in vacuo and purifiedby flash column chromatography with hexane/ethyl acetate (10:1,v/v) to afford corresponding products.
References:
Xu, Xiangchao;Ai, Yao;Wang, Rongzhou;Liu, Liping;Yang, Jiazhi;Li, Feng [Journal of Catalysis,2021,vol. 395,p. 340 - 349]